Diffusion: Definition, Meaning, Functions, Types, Topics, Example
Diffusion is a fundamental process in biology involving the movement of molecules from an area of higher concentration to an area of lower concentration. It plays a crucial role in processes like gas exchange, nutrient absorption, and waste elimination in living organisms. This passive process requires no energy and is essential for maintaining cellular homeostasis. In this article, the definition of diffusion, basic principles of diffusion, types of diffusion, factors affecting diffusion, examples of diffusion, causes of diffusion, and significance of diffusion are discussed. Diffusion is a topic of the chapter Transport in Plants in Biology.
This Story also Contains
- Definition of Diffusion
- Basic Principles of Diffusion
- Types of Diffusion
- Factors Affecting Diffusion
- Examples of Diffusion
- Causes of Diffusion
- Significance of Diffusion

Definition of Diffusion
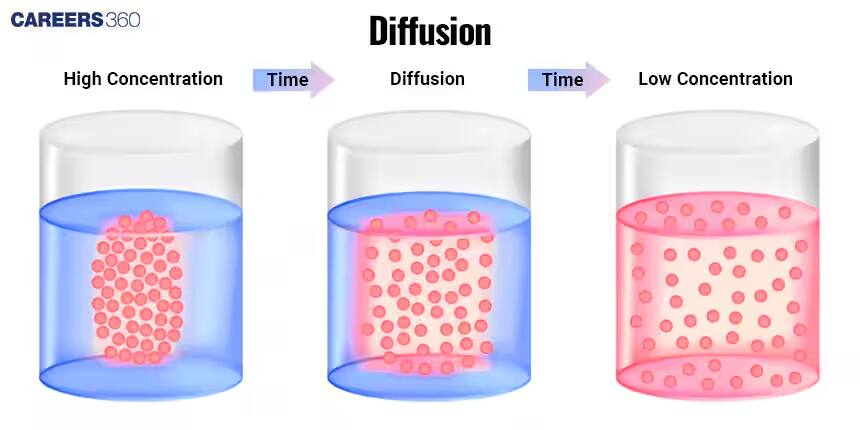
The diffusion process is characterized by the transfer of particles, molecules, or ions from an area of high to one of low concentration, following a concentration gradient. Diffusion continues until it reaches equilibrium, and then the concentration of those particles in that space is uniform. This is a fundamental physical process that transfers substances across cell membranes and within cell compartments, which event plays a key role in a variety of biological and chemical events.
Basic Principles of Diffusion
The basic principle of the diffusion process includes:
Molecular Motion and Diffusion
Particles, as stated above, are in constant and random motion. This random movement of molecules is called Brownian motion. It is this random motion that leads to the slow, progressive dispersion of particles from higher to lower concentration areas.
Concentration Gradient
This is a difference in the concentration of particles in two regions. The concentration gradient is, therefore, the driving force behind diffusion, with movement from areas of high to low concentrations to equate the concentration.
Equilibrium
The equilibrium in the process of diffusion refers to the state when the concentration of the particles within a space has become uniform. The rate of movement in one direction then becomes equal to the rate of movement in the other direction and is called dynamic equilibrium—no net movement but continued molecular motion.
Types of Diffusion
The different types of diffusion are:
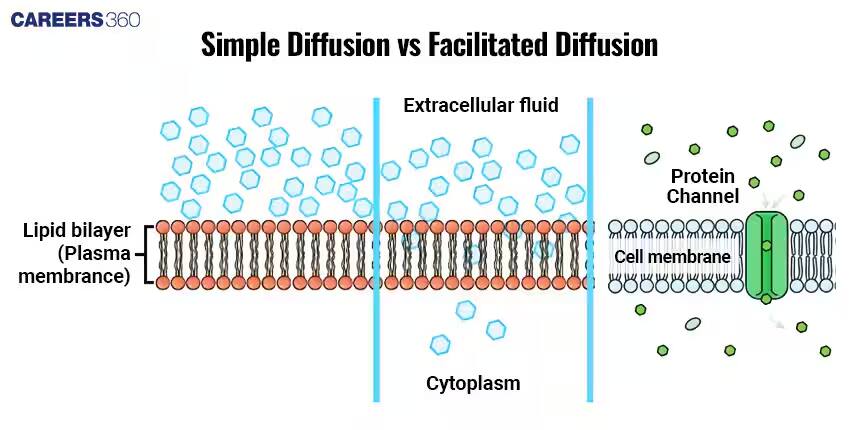
Simple Diffusion
During simple diffusion, molecules travel straight across a semipermeable membrane from an area of higher to that of a lower concentration without any energy or transport proteins. It is through simple diffusion that the exchange of oxygen and carbon dioxide happens in the lungs. These gases diffuse across the cell membranes to balance out the gases in the bloodstream and the alveoli.
Facilitated Diffusion
Role of transport proteins (channels and carriers)
Some transport proteins of the membrane facilitate facilitated diffusion, causing the motion of molecules from high to low-concentration areas. This kind of transport mechanism is applied in the case of those molecules which cannot easily travel through the lipid bilayer.
Examples (e.g., glucose transport in cells)
An example includes glucose, which goes into the cells being transported via carrier proteins. The channels and carriers, in turn, provide a way for these molecules to cross efficiently across the membrane.
Osmosis
Osmosis is a unique form of diffusion in which water molecules move through a semi-permeable membrane from an area with a low concentration of solutes to an area with a high concentration of solutes.
It is central to the maintenance of cellular homeostasis. Below, solutions are categorized by their relative concentrations of solutes: isotonic solutions have equal concentrations of solutes inside and outside the cell, so no net movement of water occurs.
A hypertonic solution has a higher concentration of solutes outside the cell than inside, resulting in possible shrinkage of the cell as water leaves it; and lastly, a hypotonic solution has a lower concentration of solutes outside the cell than inside it, and water enters the cell with the result of possible swelling or bursting of cells.
Factors Affecting Diffusion
Diffusion is affected by the following factors:
Concentration Gradient
This is the difference in concentration of some substance in two different regions. The greater the difference in concentration—that is to say, the steeper the gradient—then the faster the molecules will move from an area of high to an area of low concentration in attempts at achieving equilibrium.
Temperature
The temperature increases the kinetic energy of molecules; hence, raising the temperature increases the speed of the molecules. While the molecular speed increases, so does the rate of diffusion. With a temperature rise, there will, generally, be a faster rate of diffusion since these molecules would hit each other more often and would thus spread faster.
Particle Size
Smaller molecules diffuse faster than larger ones since of their smaller size, they meet less resistance while diffusing across the medium. The larger the molecule, the less its ability to easily pass through the spaces in whatever medium is used for diffusion, hence a slower diffusion rate.
Medium
The nature of the medium through which diffusion takes place affects the rate too. In gases, the rate is higher because their density is lower and the movement of their molecules is more as compared to liquids and solids. In liquids, the rate is slow because the molecules are more closely packed and thus face more resistance.
Examples of Diffusion
- When a tea bag is submerged in hot water, the water's colour changes as the tea bag diffuses into it.
- We may detect the odour by the way a perfume or room freshener spray diffuses into the air.
- Without stirring, sugar dissolves uniformly and sweetens the water.
- The smoke from the incense stick diffuses into the air and fills the space as we light it.
- When boiling water is added to dry noodles, the water diffuses and rehydrates the noodles, giving them a plumper, more saturated texture.
Causes of Diffusion
Diffusion is a physical and natural phenomenon that occurs naturally without the solutions being shaken or stirred. Diffusion occurs in liquids and gases because molecules can flow at random. The molecules alter their course as they collide with one another.
Significance of Diffusion
One significant process involved in various life processes is diffusion. It is the net movement of particles, ions, molecules, solution, etc., as was previously stated. Diffusion is a crucial component of the movement of molecules during cellular metabolism in all living things.
The following justifies the significance of diffusion:
This process aids in the diffusion of carbon dioxide gas into the bloodstream through the cell membrane during respiration.
In plant cells, diffusion also takes place. Water from the soil permeates all green plants through the cells that make up their root hairs.
Diffusion is the process by which ions migrate between neurones to produce electrical charge.
Also Read-
Recommended video for Diffusion
Frequently Asked Questions (FAQs)
Osmosis is a kind of diffusion in which water gets moved through a semi-permeable membrane from low to high solute concentration. The relation it has with the process of diffusion is that it is also governed by the very principle of moving substances from high to low concentration, but it deals specifically with water movement.
Diffusion finds its use in medicine, wherein it deals with the various processes connected to drug delivery systems. The drugs diffuse across the membranes to their target areas. The same happens in dialysis, where waste products diffuse through a semipermeable membrane to be removed from the blood. Diffusion enables the development of effective treatments and therapies that deal with how this transport mechanism works.
Molecules move down a gradient, flowing from an area of higher to an area of lower concentration until they reach equilibrium. This is one of the most basic mechanisms by which substances move across cell membranes and through cellular environments
Two major types of diffusion are simple diffusion and facilitated diffusion. Simple diffusion implies the direct movement of molecules through the lipid bilayer; this includes gases like oxygen and carbon dioxide. Transport proteins are needed in the membrane to aid the movement of certain molecules, such as glucose, across the membrane during facilitated diffusion.
Temperature impacts the rate of molecular motion which affects the rate of diffusion. With increasing temperature, molecular movement becomes faster, and hence, the rate of diffusion increases. On the contrary, a fall in temperature slows molecular movement and decreases the rate of diffusion.
.jpg)