Ammonium Persulfate: Introduction, Structure, Properties, Production, Uses
Ammonium persulfate or APS is defined as an inorganic compound with the chemical formula that is ![]() .
.
This Story also Contains
- Introduction
- Properties Of Ammonium Persulfate
- Production Of Ammonium Persulfate
- Health Hazards Of Ammonium Persulfate
- Uses of Ammonium persulfate

Ammonium persulfate is a colourless salt and this is basically highly soluble in water and much more than potassium salt.
Ammonium persulfate is commonly known as a strong oxidizing agent because it is commonly used in polymer chemistry or as a cleaning and bleaching agent and as an etchant.
Introduction
Ammonium persulfate is also known as ammonium peroxydisulfate, diammonium persulfate, or diammonium peroxydisulfate.
Ammonium persulfate is prepared by using the method of electrolysis with a cold concentrated solution of either of these substances like ammonium sulfate or ammonium bisulfate because of the presence of sulfuric acid with a very high current density.
Ammonium persulfate is an inorganic compound and it is also known as Ammonium peroxydisulfate Diammonium persulfate or Diammonium peroxydisulfate and it also has a wide range of applications in polymer chemistry for example as a bleaching agent, cleaning, and an etchant, etc.
On the other hand, Diammonium peroxy disulfate is an odourless crystalline solid and it is white in colour. It is readily soluble in water and plays a strong oxidizing agent role.
Properties Of Ammonium Persulfate
Ammonium persulfate structure –
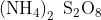
Chemical data of Ammonium persulfate -
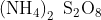
The molecular weight of Ammonium persulfate is 228.18 g/mol
The density of Ammonium persulfate is 1.98 g/cm3
Flashpoint (FP) of Ammonium persulfate is Non-flammable
Melting Point (MP) of Ammonium persulfate is 120 °C
Production Of Ammonium Persulfate
Let us see how ammonium persulfate is prepared.
Ammonium persulfate can be derived by the method of electrolysis of ammonium sulfate and dilute sulfuric acid and then it is crystallized.
The electrolytic process of ammonium sulfate and sulfuric acid formulates is to form a liquid electrolyte that is decontaminated by the electrolysis process. H2SO4 can also discharge and can generate the peroxy disulfate acid in the anode which can react with ammonium sulfate to generate ammonium persulfate. Ammonium persulfate first goes through the processes of filtration then crystallization, and then centrifugal separation, and at last, drying to get the ammonium persulfate product when the content reaches a certain concentration in the anode.
Ammonium peroxydisulfate is obtained by the process of electrolysis with a cold concentrated solution of ammonium bisulfate or by ammonium sulfate in sulfuric acid (the chemical formula is H2SO4) at a high density.
The first method was described by Hugh Marshall.
Health Hazards Of Ammonium Persulfate
Inhaling Diammonium persulfate can cause mild toxic effects. Contact with dust irritates the eyes and causes skin rashes.
When this Ammonium peroxydisulfate compound is heated toxic oxides of sulfuric acid and nitrogen fumes can be liberated.
Ammonium peroxydisulfate is a non-combustible compound but can accelerate the combustion of other substances.
Ammonium peroxydisulfate also produces irritating toxic fumes in a fire which have high explosion risks when it comes to contact with reducing agents or combustible substances.
Uses of Ammonium persulfate
Ammonium persulfate compound is used in hydrogen peroxide bleaching as an additive.
Ammonium persulfate is also used in printed circuit boards.
Ammonium persulfate is used in olefin polymerization as an initiator.
Ammonium persulfate is used in photography.
It is used as an additive to preserve food.
Ammonium persulfate is used to wash infected yeast.
Ammonium persulfate is used to remove pyrogallol stains and used as a depolarizer in batteries.
Ammonium persulfate is used as a common ingredient in hair bleach.
Frequently Asked Questions (FAQs)
Ammonium persulfate is classified as Oxidizing solid which comes under Hazard class (Category 3).
Yes, ammonium persulfate is toxic because Inhaling ammonium persulfates can cause mild toxic effects and contact with dust can irritate the eyes and cause skin rashes. Higher exposure causes a build-up of fluid in the lungs in pulmonary edema , and needs a medical emergency.
The chemical formula of Ammonium persulfate is ![]() .
.
By dissolving 1 gm of ammonium persulphate in 10 mL of water we can get 10% of ammonium persulphate.
Fresh solutions of ammonium persulfate product can be prepared for the most effective use in electrophoresis and the solutions stored at room temperature are not stable even if they are protected from light or air.
Storage of solutions should be at 2-8 °C which will allow their use for up to 12 hours.