Biomolecules - Types, Structure and Function
Biomolecules are complex lifeless chemical substances that we get from living tissues. Biomolecules are the organic compounds that are essential for the maintenance of metabolic processes. Biomolecules not only build up the living cells but also help in their growth, development, and ability to reproduce. Examples of biomolecules are carbohydrates, proteins, lipids, and nucleic acids.
This Story also Contains
- Important Topics Of Biomolecules
- Overview Of Biomolecules
- Proteins
- Amino acids
- Nucleic acids
- How To Prepare For Biomolecules?
- Book for Biomolecules
- Important PYQs
- Practice more questions from the link given below:
- Conclusion:
This article will give you all the information regarding the biomolecules chapter. This will help you to make your strategies in your preparation and it will also guide you in how to prepare for this chapter and the best-prescribed books.
Important Topics Of Biomolecules
Classification Of Carbohydrates And Its Structure:
Carbohydrates are composed of carbon, hydrogen, and oxygen; the general formula is Cx(H2O)y. They are categorized based on their degree of polymerization as monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides consist of the simplest units of carbohydrates, which cannot be further hydrolyzed into still smaller carbohydrate molecules. Two monosaccharides are then linked together via a condensation reaction to form a single disaccharide. Oligosaccharides and polysaccharides are chains of monosaccharide units.
Tests For Carbohydrates:
Several chemicals are used in the tests for carbohydrates because of the peculiar characteristics of each compound. Because Each body is specific for a certain type of carbohydrate such as the benedict test is applicable in determining reducing sugars, for example, glucose and fructose, iodine test is designed for identifying starch, whereby an iodine solution changes to a blue-black color on coming into contact with a sample of the starch.
Properties Of Glucose:
Glucose exhibits a couple of Properties, which make it suitable to play the above-mentioned biological roles. First, it is known as a reducing sugar in that it has the ability to reduce other oxidizing agents. Glucose is oxidized with nitric acid, saccharic acid is formed. Saccharic acid is also known as glucaric acid.
Amino Acids:
Amino Acids are the very building blocks of proteins, and proteins are the most fundamental macromolecules in any living organism. These organic compounds perform critical functions not only towards the structure of cells but also in various physiological activities, as diverse as muscle contraction and the activation of the immune system.
Proteins:
Proteins are large, complex molecules consisting of chains of amino acids. Amino acids are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and on rare occasions, sulfur. They have vital roles in structure, function, and regulation in the body's tissues and organs.
Enzymes:
Enzymes are specialty proteins that function as catalysts in biochemical reactions—that is, they speed up reactions but they are not consumed in the reaction. Each enzyme is specific to one reaction or type of reaction. It often binds to a substrate, the molecule upon which the enzyme acts. This interaction lowers the activation energy required for the reaction to proceed.
Vitamins:
Vitamins are organic materials that the body requires for proper metabolism and which are only required in trace amounts. They take part in many functions of our bodies: starting with support to our immune system and ending with the production of energy.
Overview Of Biomolecules
Types of Biomolecules
There are four types of biomolecules:
- Carbohydrates
- Lipids
- Proteins
- Nucleic acids
Carbohydrates
Carbohydrates are the optically active polyhydroxy aldehydes or polyhydroxy ketones or substances that produce them on hydrolysis. Carbohydrates play a vital role in our daily life. They provide us the the three basic necessary things of life which are food (starch), clothing (cellulose), and shelter (cellulose).
Classification of Carbohydrates
Carbohydrates are also known as the saccharides. And classified into three categories
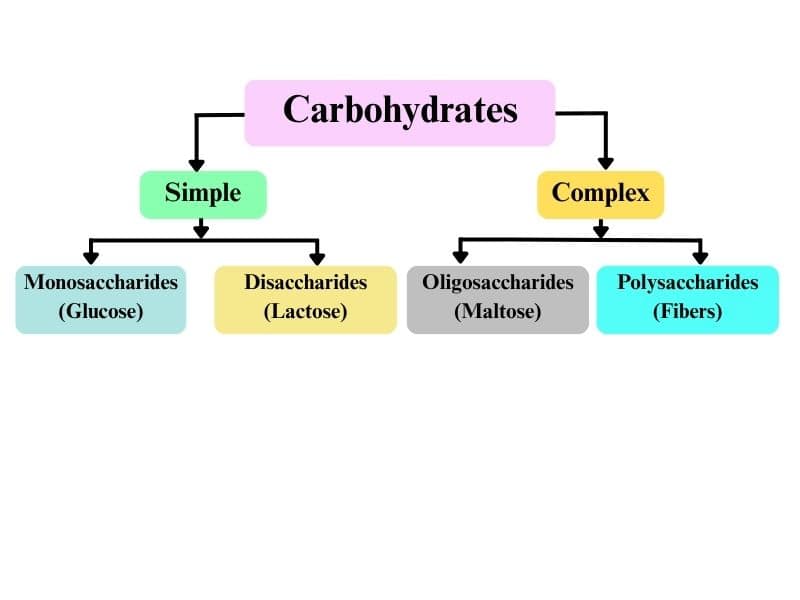
- Monosaccharide
- Oligosaccharides
- Polysaccharides
Monosaccharides
It cannot hydrolysis to the smaller molecules. Their general formula is (CH2O)n where n= 3-7.
Oligosaccharides
These on hydrolyzed give 2-10 molecules of monosaccharides like Disaccharides, Trisaccharides, and Tetrasaccharides.
Polysaccharides
Carbohydrates in hydrolysis give a large number of monosaccharide units such as Starch, cellulose, and glycogen.
Reducing and Non-reducing Sugars
Those sugars that reduce Fehling's and Tollens's solutions are called reducing sugars and those that do not reduce these reagents are called non-reducing sugars. All the monosaccharides and disaccharides, except sucrose, are reducing sugars, whereas all the polysaccharides are called non-reducing carbohydrates.
Cyclic Structure of Carbohydrates
The CHO group of glucose either reacts with the C-5 OH group or the C-4 OH group to give hemiacetalic linkage and forms stable six- and five-membered cyclic rings, respectively. If the CHO group reacts with the C-6 OH group, then a less stable seven-membered ring will form, or with C-3 OH, a four-membered ring will be formed, both are unstable.
Haworth representation: The cyclic structure of glucose was established by English chemist W.N. Haworth. The cyclic structure of glucose is depicted below.

Proteins
Proteins are the condensation polymer of the alpha-amino acids. They are present in almost all the living cells of plants and animals. In human beings, proteins are the main components of muscles, hairs, nails, tendons, arteries, and connective tissues. Each living cell is made up of thousands of different proteins.
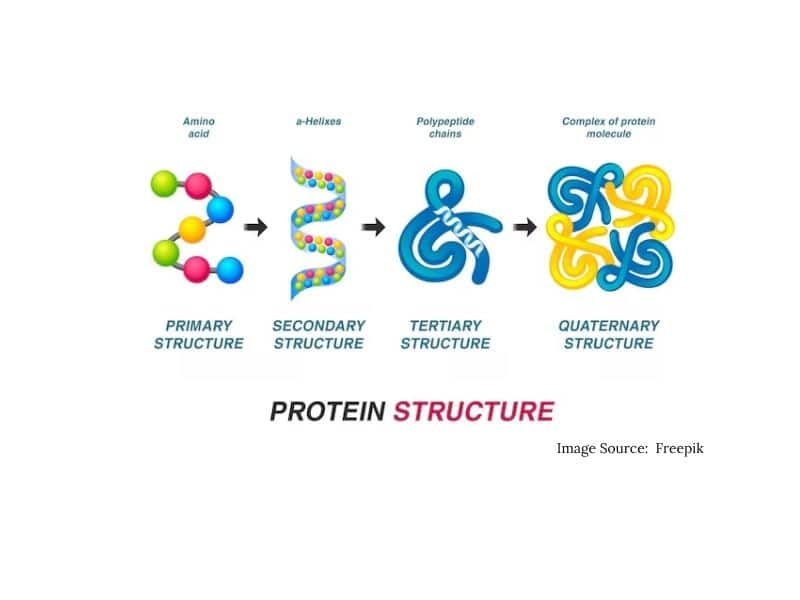
Structure of Proteins
1) Primary Structure -Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein. Any change in this primary structure i.e., the sequence of amino acids creates a different protein.
2) Secondary Structure- Two types of secondary structure are alpha helix and beta plotted sheet structure which are held by hydrogen bonds.
3) Tertiary Structure -Tertiary structure is the interaction between protein molecules in a 3D manner which involves a disulfide bond, Hydrogen bond, and Ionic bond.
4) Quaternary Structure - Quaternary structure is an association of protein molecules in close arrangement.
The diagrammatic representation of all these structures is shown below:
Amino acids
Amino acids contain amino (-NH2) and carboxyl (-COOH) as functional groups. Depending upon the relative positions of the -NH2 group w.r.t -COOH group, the amino acids are classified as alpha, beta, gamma, delta, etc. amino acids. However, only alpha amino acid is obtained during the hydrolysis of protein. so that alpha amino acid is the building block of proteins
There are 20 amino acids in almost all proteins and these determine the properties of amino acids
Amino acids have amino(-NH2) and carboxyl(-COOH) functional groups. Based on the relative position of the two functional groups in the alkyl chain, the amino acids are categorized as α, β,? δ and so on. On the hydrolysis of proteins, only α-amino acids are formed. Amino acids may also contain other functional groups.

- Amino acids which can be synthesized in our body are known as non-essential amino acids while those that can not be synthesized in our body are known as essential amino acids.
- They are usually colorless, water-soluble, high melting and crystalline solids.
- Except glycine, all other naturally occurring amino acids are optically active.
- Most naturally occurring amino acids have L-configuration.
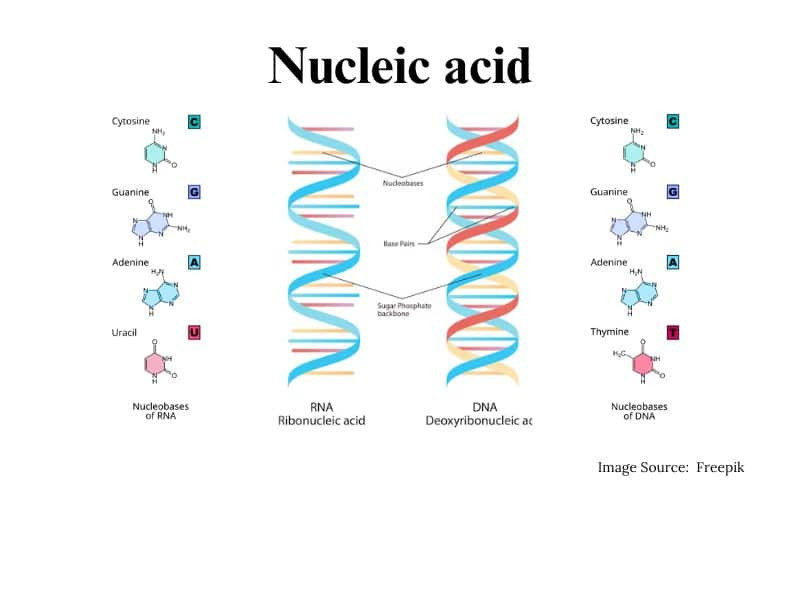
Nucleic acids
Nucleic acid is the polymer present in the living system. Nucleic acids are also called polynucleotides because their repeating unit is a nucleotide. Nucleotides contain three parts i.e. a sugar molecule, a nitrogenous base, and a phosphoric acid.
Structure of Nucleic acids
The structure of nucleic acid contains:
- A pentose sugar: pentose sugar in DNA is called deoxyribose
pentose sugar in RNA is called ribose
- Nitrogenous base: 1) Purines
2) Pyridines
- Nucleoside and Nucleotide: Nucleosides contain pentose sugar and nitrogenous base
Nucleotides contain a sugar, base, and a phosphate group
A double helix consists of two strands that are antiparallel and complementary to each other. Two helixes are held together by hydrogen bonds.
Function of biomolecules
Biomolecules are essential components of life and perform various functions such as:
- Carbohydrates are the source of energy they store energy and help in the recognition of cells
- Proteins help in the transportation of molecules across the cell membranes
- Lipids form the cellular membrane and provide insulation and protection to the organs.
- Nucleic acids store the genetic information and help in protein synthesis.
How To Prepare For Biomolecules?
-
Focus on core biomolecule classes—carbohydrates, proteins, lipids, nucleic acids, enzymes and vitamins. Know their structures, classification, function, and biological significance.
-
Memorize essential structures like glucose, fructose (including cyclic forms), essential amino acids, and nucleotide components. Focus on one or two key sugars and amino acids to streamline recall.
-
Master enzyme behavior and kinetics: understand factors influencing enzyme activity, inhibition types, and how enzymes regulate reaction rates—they’re common in JEE questions.
-
Practice regularly with past JEE Main and JEE Advanced questions from biomolecules. Use mock tests to identify weak points and improve speed, accuracy, and confidence.
Book for Biomolecules
For this chapter, first, you need to finish the important topics of biomolecules class 12 thoroughly from the class 12th NCERT book and then simultaneously solve the examples and questions given in the book.
Important PYQs
Question 1: Given below are two statements :
Statement (I) : On hydrolysis, oligo peptides give rise to fewer number of α-amino acids while proteins give rise to a large number of β-amino acids.
Statement (II) : Natural proteins are denatured by acids which convert the water soluble form of fibrous proteins to their water insoluble form.
In the light of the above statements, choose the most appropriate answer from the options given below :
1) Both statement I and statement II are correct.
2) Statement I is incorrect but Statement II is correct.
3) Both statement I and statement II are incorrect.
4) Statement I is correct but Statement II is incorrect.
Solution:
Protein give rise to alpha amino acid, so the above statement is incorrect.
Natural proteins are denatured by acid. Due to this, globules unfold and helices get uncoiled.
Hence, the correct answer is option (3).
Question 2:
Given below are two statements :
Statement-I: D-(+)- Glucose and D-(+)- fructose are formed on hydrolysis of sucrose.
Statement II : Invert sugar is formed during sucrose hydrolysis.
In the light of the above statements, choose the correct answer from the options given below -
1) Both Statement I and Statement II are true.
2) Statement I is false but Statement II are true.
3) Statement I is true but Statement II is false.
4) Both Statement I and Statement II are false.
Solution:
On hydrolysis of sucrose gives D−(+)-glucose and D-(-)-fructose while in St. (1) D-(+)-fructose is given; hence, Statement-(1) is incorrect.

St. II - It is correct because sucrose on hydrolysis gives invert sugar
Hence, the correct answer is option (2).
Practice more questions from the link given below:
1)
Evidence for Open Chain Structure of Glucose -Practice questions and MCQs
2) Disaccharides and Polysaccharides-Practice questions and MCQs
Conclusion:
Biomolecules—including carbohydrates, proteins, lipids, enzymes, nucleic acids, and vitamins—form the chemical foundation of life. They serve key roles in energy storage, structural support, catalysis, genetic information transfer, and cell signaling. This chapter is essential for Class 12 chemistry and serves as a scoring yet manageable topic in JEE Main and Advanced, typically contributing one 3–4 mark question. Mastery of NCERT fundamentals, focused revision, and solving previous-year problems make it a high-yield area with minimal effort.
Frequently Asked Questions (FAQs)
Carbohydrates serve as a primary energy source for cells, are involved in cell recognition and signaling, and provide structure in plants (as cellulose) and some fungi. They can also store energy in the form of glycogen in animals.
Nucleic acids are long chains of nucleotides, that consist of a sugar, a phosphate group, and a nitrogenous base. DNA is typically double-stranded, forming a double helix, while RNA is usually single-stranded.
Studying biomolecules is important for understanding the fundamental processes of life, including metabolism, genetic information transfer, and cellular function. Also biomolecular research can lead to advancements in medicine, biotechnology, and our understanding of diseases.
Lipids are a diverse group of hydrophobic molecules, including fats, oils, waxes, and steroids.
The four main types of biomolecules are:
- Proteins: Made of amino acids, proteins are crucial for the structure, function, and regulation of the body’s tissues and organs.
- Nucleic Acids: DNA and RNA are responsible for the storage and transmission of genetic information.
- Carbohydrates: Composed of sugar molecules, carbohydrates are a primary source of energy for cells and play roles in cell structure.
- Lipids: These are fats and oils that store energy, provide insulation, and makeup cell membranes.