Chemical Kinetics - Notes, Topics, Formula, Books, FAQs
Have you ever wondered why some reactions occur instantly, like the explosion of fireworks, while others, like rusting of iron, take months or years? How do catalysts speed up reactions without being consumed? You will get all these answer by studying this chapter chemical kinetics. It is the branch of chemistry that deals with the rate of chemical reactions, factors affecting them, and the mechanism by which they occur.
This Story also Contains
- Important Topics Of Chemical Kinetics
- Overview of the Chemical Kinetics
- Prepartion Tips for Chemical Kinetics
- Prescribed Books For Chemical Kinetics
- Previous Year Questions of Chemical Kinectics
- Conclusion:
Chemical kinetics is a part of chemistry that focuses on how fast chemical reactions happen. Some reactions are super quick like mixing silver nitrate $\left(\mathrm{AgNO}_3\right)$ with hydrochloric acid (HCl) to make silver chloride (AgCl). Other reactions take a very long time, such as a diamond slowly turning into graphite. Besides whether a reaction can happen, factors like how fast it goes and various other conditions affect its speed. In this chapter, you'll study how reaction rates work, what reaction order means, the Arrhenius equation, collision theory, and more.
Important Topics Of Chemical Kinetics
Rate Of Chemical Reaction:
The rate at which products are formed in the course of a reaction is called the Rate Of Reaction. It is a universal fact that there exists a wide extent of variation in rates in all chemical reactions. Of the chemical reactions, some take place almost instantaneously, while others, in general, take some time to reach the final equilibrium.
For a reaction:
$a A+b B \rightarrow c C+d D$
The rate can be expressed as:
$\text { Rate }=-\frac{1}{a} \frac{d[A]}{d t}=-\frac{1}{b} \frac{d[B]}{d t}=\frac{1}{c} \frac{d[C]}{d t}=\frac{1}{d} \frac{d[D]}{d t}$
Elementary And Complex Reactions:
An elementary reaction is a one-step process where reactants directly go to form products. Example
$\mathrm{NO}+\mathrm{O}_3 \rightarrow \mathrm{NO}_2+\mathrm{O}_2$ (Single step reaction)
However, complex reactions that occurs in two or more steps (sequence of elementary steps).The overall reaction is the sum of all elementary steps. Example:
Decomposition of hydrogen peroxide:
$2 \mathrm{H}_2 \mathrm{O}_2 \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2$ (Occurs in multiple steps involving free radicals).
Order Of Reaction:
The order of reaction quantifies how the concentration of reactants affects the speed of a chemical reaction. In simpler terms, it is the sum of the powers to which the concentration terms are raised in the rate law expression.
For a reaction:
$a A+b B \rightarrow \text { Products }$
Rate law:
$\text { Rate }=k[A]^x[B]^y$
Here,
$x=$ order w.r.t A
$y=$ order w.r.t B
Overall order $=x+y$
Zero-Order Reactions:
Zero-order reactions are especially important in catalysis when the reaction rate is limited by the number of active sites on a catalyst, and not by the concentration of reactants. Rate of reaction of Zero Order Reaction is independent of concentration of the reactants.
For a reaction:
$A \rightarrow \text { Products }$
If it is zero-order in $A$ :
$\text { Rate }=k[A]^0=k$
Here, $k=$ rate constant (units: $\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}$ ).
Rate is constant and does not change with concentration.
First-Order Reactions:
First Order Reaction describe a scenario where the rate of reaction is directly proportional to the concentration of only one reactant. The rate of the reaction is proportional to the first power of the concentration of the reactant
Second-Order Reactions:
The case in which the rate is proportional to the product of two concentrations of two reactants or to the square of the concentration of a single reactant is called a Second Order Reaction.
Pseudo-First-Order Reactions:
A Pseudo First Order Reaction is a complex process involving more than one reactant or including complicated steps; however, it apparently obeys the first-order kinetics. Under certain conditions of the experiments, it simplifies to a first-order rate law, which usually happens when the concentration of one reactant is in large excess or if reaction intermediates are involved.
Example:
Hydrolysis of ester in excess water
$\mathrm{CH}_3 \mathrm{COOC}_2 \mathrm{H}_5+\mathrm{H}_2 \mathrm{O} \xrightarrow{\mathrm{H}^{+}} \mathrm{CH}_3 \mathrm{COOH}+\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}$
Actually second-order ([ester][water])
But since $\left[\mathrm{H}_2 \mathrm{O}\right] \gg[$ ester $]$, it becomes pseudo-first-order.
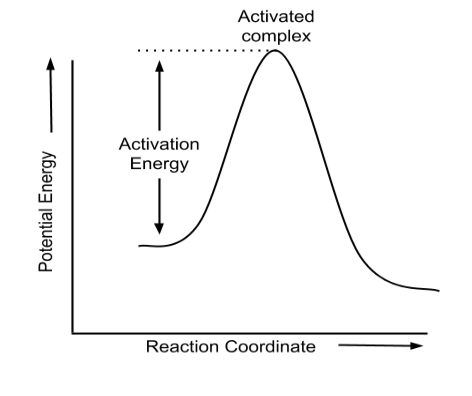
Arrhenius Equation:
The Arrhenius equation is a chemical kinetics equation that describes how the rate of a chemical reaction depends on temperature. It is also used to understand how increasing the temperature or decreasing the activation energy increases the rate of a reaction.
$k=A e^{-\frac{E_a}{R T}}$
Where
$k=$ rate constant
$A=$ frequency factor (collision frequency + proper orientation)
$E_a=$ activation energy $(\mathrm{J} / \mathrm{mol}$ or $\mathrm{kJ} / \mathrm{mol})$
$R=$ gas constant $\left(8.314 \mathrm{~J} \cdot \mathrm{~mol}^{-1} \cdot \mathrm{~K}^{-1}\right)$
$T=$ absolute temperature (K)
Molecularity Of Reaction:
Molecularity is referred to as the number of reactant molecules coming together simultaneously to collide with each other and enter into the transition state that finally leads to a chemical reaction. This approach is used to describe theoretically the mechanism of elementary reactions.
Rate Law:
The rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it.
$\text { Rate }=k[A]^m[B]^n$
Where
Rate = rate of reaction
$k=$ rate constant (specific for a reaction at a given T)
$[A],[B]=$ concentrations of reactants
$m, n=$ reaction orders (can be $0,1,2$, fractional, or even negative)
Nth Order Reaction:
The nth-order reaction is one in which the rate of the reaction depends upon the concentration of one or more reactants raised to some power, which is called the order of the reaction. The order of a reaction may be an integer or even a fraction and is generally considered representative of how the rate of reaction depends on the concentration of reactants. Mathematically, this rate law may be defined for an nth-order reaction by the expression given below:
Rate=k[A]n
Overview of the Chemical Kinetics
(i) Rate of reaction: Similar to any object moving with a certain velocity, chemical reactions also occur with certain velocities also known as the rate of reaction. This rate of reaction is defined as how fast the product is forming or how fast the reactants are getting consumed with respect to time. Mathematically, it can be expressed as follows:
$\Delta [R] \,=\, [R]_{2}\, -\, [R]_{1}$
$\Delta[P]=[P]_2-[P]_1$
$\Delta t \, =\,t_{2}\, -\, t_{1}$
$\therefore\: Rate\, of\, reaction = \frac{Increase\, in\, concentration\, of\, P}{Time\, \, taken} = \frac{Decrease\, \, in\, concentration\,of\, R}{Time\,taken}$
$\therefore\: Rate\, of\, reaction = \frac{\Delta P}{\Delta t} = \frac{-\Delta R}{\Delta t}$
(ii) Rate expression and rate constant: For the general reaction:
$aA\, +\, bB\rightarrow cC\,+dD$
The rate equation is given as follows:
$Rate\,\alpha [A]^{x}\,[B]^{y}$
This equation can be written as follows:
$Rate\,=k[A]^{x}\,[B]^{y}$
where x and y may or may not be stoichiometric coefficients. The addition of these components (x+y) gives the order of the reaction.
(iii) Order of the reaction: The rate equation of a chemical reaction is given as:
$Rate\,=k[A]^{x}\,[B]^{y}$
where (x+y) gives the order of the reaction and k is the rate constant.
-
Units of rate constant: For a general reaction:
$aA\, +\, bB\rightarrow cC\,+dD$
$Rate\,=k[A]^{x}\,[B]^{y}$
$k\, =\, \frac{Rate}{[A]^{x}[B]^{y}}$
The unit of rate constant (k) depends on the order of the reaction i.e. (x+y).Reaction type
Order of reaction
Units of rate constant
Zero-order reaction
0
$\mathrm{mol} \mathrm{L}^{-1} \mathrm{~s}^{-1}$ First order reaction
1
$$
s^1
$$Second order reaction
2
$\mathrm{mol}^{-1} \mathrm{~L} \mathrm{~s}^{-1}$
Zero-order reaction: It is the type of reaction, where the order of reaction is proportional to the zero power of the concentration of reactants. The rate equation is given as follows:
$[R]\,=\,-kt\,+\,[R]_{0}$
-
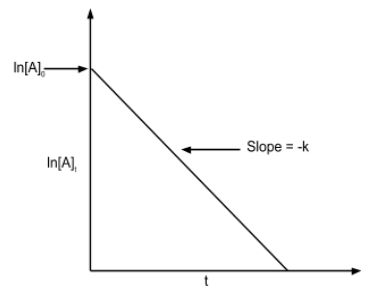
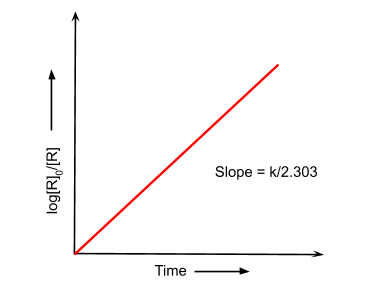
First order reaction: In these type of reactions, the rate of reaction is proportional to the first power of the concentration of reactants. The rate equation is given as follows:
$log\frac{[R]_{0}}{[R]}\,=\,\frac{k(t_{2}-t_{1})}{2.303}$
-
The half-life of reaction: It is the time at which the concentration of reactant is half of its initial value. It is denoted by t1/2. Mathematically, it can be described as follows:
$$t_{1/2}\, =\, \frac{0.693}{k}$$
-
Arrhenius Equation: The rate of a chemical reaction depends on the temperature. For every 100 rise in temperature, the rate constant gets doubled. This temperature dependency of the rate of chemical reaction is explained by the Arrhenius equation as given below:
k = A e-Ea/RT
where A is the Arrhenius factor, Ea is the activation energy and R is the gas constant
-
Collision Theory of Chemical Reactions: This theory gives a deeper insight into the mechanism of the chemical reactions. According to this theory, the reactant molecules are considered to be spherical molecules and the reaction occurs when these spherical molecules collide with each other. Mathematically, it can be described as follows:
Rate = $\mathrm{Z}_{\mathrm{AB}} \mathrm{e}^{\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}$
where $\mathrm{Z}_{\mathrm{AB}}$ is the collision frequency. The number of collisions per second per unit volume of the reaction mixture is known as "collision frequency(Z)".
Prepartion Tips for Chemical Kinetics
-
Master the Basics: Understand core concepts like reaction rate, order of reaction, rate constant, and key equations (e.g., Arrhenius equation, rate laws).
-
Focus on Key Topics: Study how concentration, temperature, and catalysts affect reactions, and practice deriving integrated rate laws for different reaction orders.
-
Practice and Solve Problems: Regularly solve problems from textbooks and previous exams, focusing on both theoretical concepts and numerical calculations.
-
Understand Real-World Applications: Relate theory to practical scenarios in industries, medicine, and daily life, while also managing your time efficiently for consistent revision.
In everyday life, there are various important chemical reactions which occur around us as follows:
-
Chemical kinetics helps explain how refrigerators slow down food spoilage. By lowering temperature, the kinetic energy of molecules drops, leading to fewer and less energetic collisions—so fewer reactions occur, and microbes work more slowly.In essence, chilling food reduces the rate of chemical and enzymatic processes (like oxidation or enzymatic browning), keeping your groceries fresh for longer.

-
Each popcorn kernel holds a tiny bit of water and starch, all sealed within a rigid shell. When heated, the water turns into steam, increasing pressure inside the kernel. Once this pressure reaches a critical point—around 180 °C (≈356 °F)—the shell bursts open. The sudden release of steam causes the starch inside to expand rapidly, turning the kernel inside-out with a “pop.” Oxygen cools and sets the expanded starch into the fluffy popcorn shape we enjoy.

-
In chemical kinetics, you also learn about how by changing the reaction conditions like temperature and pressure, the rate of reaction changes. It also gives you information about the reaction mechanisms, activation energy, and reaction intermediates.
Prescribed Books For Chemical Kinetics
First, you must finish the class XII NCERT book and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. This article will help you with a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Previous Year Questions of Chemical Kinectics
Question 1: Consider the following first order gas phase reaction at constant temperature
$\mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$
If the total pressure of the gases is found to be 200 torr after $23 \mathrm{sec}$. and 300 torr upon the complete decomposition of $\mathrm{A}$ after a very long time, then the rate constant of the given reaction is ________ $\times 10^{-2} \mathrm{~s}^{-1}$ (nearest integer)
$\left[\right.$ Given : $\left.\log _{10}(2)=0.301\right]$
Solution:
$ \mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g}) $
$ \mathrm{P}_{23}=\mathrm{P}_0+2 \mathrm{x}=200 $
$ \mathrm{P}_{\infty}=3 \mathrm{P}_0=300 $
$ \mathrm{P}_0=100 $
$ \mathrm{~K}=\frac{1}{\mathrm{t}} \ln \frac{\mathrm{P}_{\infty}-\mathrm{P}_0}{\mathrm{P}_{\infty}-\mathrm{P}_{\mathrm{t}}} $
$ \mathrm{K}=\frac{2.3}{23} \log \frac{300-100}{300-200} $
$ \quad=\frac{2.3 \times 0.301}{23}=0.0301=3.01 \times 10^{-2} \mathrm{sec}^{-1}$
Hence, the answer is (3).
Question 2: Consider the following single step reaction in gas phase at constant temperature.
$2 \mathrm{~A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightarrow \mathrm{C}_{(\mathrm{g})}$
The initial rate of the reaction is recorded as $r_1$ when the reaction starts with $1.5 \mathrm{~atm}$ pressure of A and $0.7 \mathrm{~atm}$ pressure of $\mathrm{B}$. After some time, the rate $r_2$ is recorded when the pressure of $\mathrm{C}$ becomes $0.5 \mathrm{~atm}$. The ratio $\mathrm{r}_1: \mathrm{r}_2$ is_________$\times 10^{-1}$. (Nearest integer)
Solution:
$2 \mathrm{~A}(\mathrm{~g})+\mathrm{B}(\mathrm{g}) \longrightarrow \mathrm{C}(\mathrm{g})$
$\begin{array}{llll}\mathrm{r}_1 & 1.5 \mathrm{~atm} & 0.7 \mathrm{~atm} & \\ \mathrm{r}_2 & 0.5 \mathrm{~atm} & 0.2 \mathrm{~atm} & 0.5 \mathrm{~atm}\end{array}$
$\begin{aligned} & \because r=K\left[P_A\right]^2\left[P_B\right] \\ & r_1=K[1.5]^2[0.7] \\ & r_2=K[0.5]^2[0.2] \\ & \frac{r_1}{r_2}=9 \times \frac{7}{2}=31.5=315 \times 10^{-1}\end{aligned}$
Hence, the answer is (315).
Question 3: What is meant by the Rate law and Rate constant of a reaction. Identify the order of a reaction if the units of its Rate constant are : $\mathrm{mol}^{-1} \mathrm{~L} \mathrm{~s}^{-1}$.
Solution:
Rate law:
A rate law is a mathematical formula that depicts the relationship between the rate of reaction and the concentrations of each component.
Rate $=k[A]^m[B]^n \cdots$
where k= rate constant, A and B are concentrations of reactant,s and m,n are orders with respect to each reactant.
Rate Constant:
It is the specific rate constant (k) that determines the proportionality between the rate of the reaction and the concentrations of the reaction's reactants and products.
- It depends on temperature, but it does not depend on the concentration of the reactant.
-Its unit depends on the overall order of reaction.
The differential rate expression for nth order reaction is as follows:
$-\frac{\mathrm{dx}}{\mathrm{dt}}=\mathrm{k}(\mathrm{a}-\mathrm{x})^{\mathrm{n}}$
$\mathrm{k}=\frac{\mathrm{dx}}{(\mathrm{a}-\mathrm{x})^{\mathrm{n}} \mathrm{dt}}=\frac{(\text { concentration })}{(\text { concentration })^{\mathrm{n}} \text { time }}=(\text { conc. })^{1-\mathrm{n}}$ time $^{-1}$
If concentration be expressed in mole L-1 and time in minutes, then$\mathrm{k}=\left(\mathrm{mole}^{-1}\right)^{1-\mathrm{n}} \min ^{-1}$
Since rate units are usually mol L−1s−1and concentration units are mol L−1, the units of k change with reaction order.
Order of Reaction:
The order of reaction is some of power of the concentration of reactants.
Rate $=k[A]^m[B]^n \cdots$
Order of reaction = m+n.....
The order of a reaction can be determined from the units of the rate constant $k$.
The units of the rate constant depend on the overall order of the reaction.
Units of $k=\mathrm{mol}^{-1} \mathrm{Ls}^{-1}$
The general form:
$
\mathrm{mol}^{1-n}=\mathrm{mol}^{-1} \Rightarrow 1-n=-1 \Rightarrow n=2
$
The order of the reaction is: 2 (Second-order)
Hence, the answer is 2
Question 4: Assertion (A) : In a first order reaction, if the concentration of the reactant is doubled, its half-life is also doubled.
Reason (R): The half-life of a reaction does not depend upon the initial concentration of the reactant in a first order reaction.
1) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
2) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
3) Assertion (A) is true, but Reason (R) is false.
4) Assertion (A) is false, but Reason (R) is true.
Solution:
For a first-order reaction, half-life $\left(t_{1 / 2}\right)$ is independent of the initial concentration.
$$
t_{1 / 2}=\frac{0.693}{k}
$$
The half-life of a reaction does not depend upon the initial concentration of the reactant in a first-order reaction. This is true.
So the Assertion is false. Doubling the concentration does not affect the half-life.
The assertion is False, but Reason is true.
Hence, the correct answer is option (4).
Practice more questions from the link given below:
Conclusion:
Chemical kinetics is crucial for understanding how chemical reactions occur and the factors affecting their rates. It helps in optimizing industrial processes, designing drugs, and controlling reactions in daily life. In exams like JEE Main, NEET, SRMJEEE, BITSAT, and WBJEE, this topic carries significant weightage due to its practical applications in various fields, including medicine and engineering. Mastery of concepts like reaction rates, the Arrhenius equation, and reaction order can boost performance in these competitive exams, making it essential for success.
Frequently Asked Questions (FAQs)
The rate constant (k) is a proportionality constant that links the rate of reaction to the concentrations of reactants. The reaction rate refers to the speed at which the reactants are converted into products.
In a first-order reaction, the rate depends on the concentration of only one reactant, while in a second-order reaction, the rate depends on the square of the concentration of one reactant or the product of the concentrations of two reactants.
As temperature increases, the rate of reaction generally increases because the molecules move faster, leading to more frequent and energetic collisions, thus overcoming the activation energy more easily.
Chemical kinetics is the study of the rates and mechanisms of chemical reactions. It involves the investigation of how fast or slow a reaction occurs and the factors that influence the reaction rate.
There are several factors that can affect the rate of a chemical reaction, including temperature, the concentration of reactants, the surface area of reactants, the presence of catalysts, and the nature of the reactants and products.
A reaction mechanism is a step-by-step process that explains how a chemical reaction occurs. It includes the intermediate species and the elementary reactions that lead to the formation of the final products.
A rate law is an equation that relates the rate of a chemical reaction to the concentration of the reactants. It provides information about the dependence of the reaction rate on the reactant concentrations and can be used to determine the reaction order and rate constant.
Chemical kinetics plays a crucial role in various fields, including chemical engineering, medicine, environmental science, and materials science. It can be used to design and optimize chemical reactions, develop new drugs and medicines, study atmospheric chemistry and pollution, and understand the behaviour of materials under different conditions.
Reversible reaction kinetics examines the rates at which chemical reactions occur in both forward and backward directions, considering factors such as concentration, temperature, and catalysts to describe the dynamic equilibrium between reactants and products