Dipole Moment - Overview, Definition, Formula, FAQs
A dipole moment is a measure of the separation of positive and negative charges in a molecule, indicating the polarity of the molecule. It arises when there is an uneven distribution of electron density, resulting in partial positive and negative charges at different ends of the molecule. The dipole moment is a vector quantity, having both magnitude and direction. The magnitude of the dipole moment is given by the product of the charge difference and the distance between the charges. It is expressed in Debye units (D).
This Story also Contains
- Dipole Moment
- Some Solved Examples
- Summary
Also read :
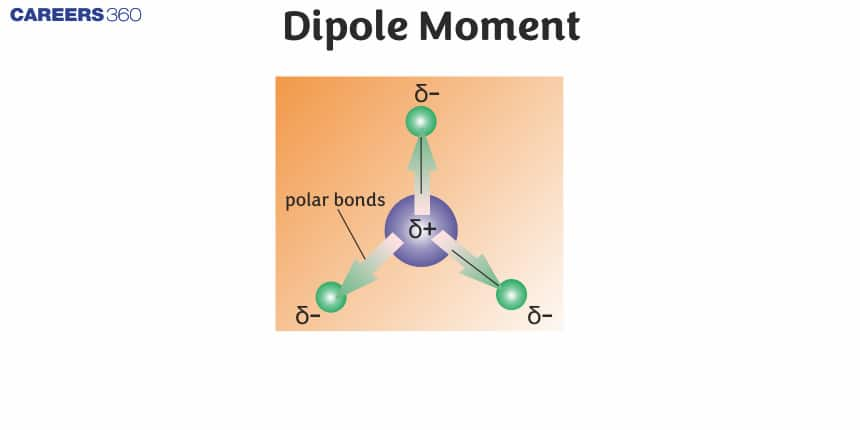
Dipole Moment
When the covalent bond forms between two different atoms then because of the difference in electronegativity the electrons get shifted towards the more electronegative atom and thus form the polar covalent bond and this polar molecule is known as a dipole molecule. The more electronegative atom occupies a partial negative charge (δ-) and the other atom possesses a partial positive charge (δ+). This separation of charge gives rise to a bond dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu (µ) and is given by the formula as shown below, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges: μ=Qr

(a) There is a small difference in electronegativity between C and H, represented as a short vector. (b) The electronegativity difference between B and F is much larger, so the vector representing the bond moment is much longer.
For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Homonuclear diatomic molecules such as Br2 and N2 have no difference in electronegativity, so their dipole moment is zero. For heteronuclear molecules such as CO, there is a small dipole moment. For HF, there is a larger dipole moment because there is a larger difference in electronegativity.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
When a molecule contains more than one bond, the geometry must be taken into account. If the bonds in a molecule are arranged such that their dipole moments cancel, then the molecule is non-polar. For example, in the case of CO2 as shown in the figure given below. Each of the bonds is polar, but the molecule as a whole is non-polar. The dipole moments cancel each other because they are pointed in opposite directions.

In the case of the water molecule, the Lewis structure again shows that there are two bonds to a central atom, and the electronegativity difference again shows that each of these bonds has a nonzero bond moment. In this case, however, the molecular structure is bent because of the lone pairs on O, and the two bond moments do not cancel. Therefore, water does have a net dipole moment and is a polar molecule (dipole).

Recommended Topic video on (Dipole Moment )
Some Solved Examples
Example 1: The most polar compound among the following is :
1)

2)

3)

4)

Solution
Resultant dipole moment -
Let XY and XZ be two polar bonds inclined at an angle θ their dipole moments are μ1 and μ2
Resultant μR=μ12+μ22+2μ1μ2cosθ
In  the bond dipole vector of the C-F bond is not subtractive.
the bond dipole vector of the C-F bond is not subtractive.
Example 2: The molecule which has zero dipole moment is :
1)CH2Cl2
2) BF3
3)NF3
4)ClO2
Solution
The dipole moment of a compound having regular geometry and the same type of atom is zero.
BF3 has a triangular planar structure hence its resultant dipole moment is zero.

Hence, the correct answer is Option (2)
Example 3: Which of the following isomers will have the highest dipole moment?
1) Ortho form
2)Meta form
3)Para form
4)All have equal dipole moment
Solution

Because the dipole moment is a vector quantity and the resultant dipole moment is highest when the angle between them is minimum.
Hence, the option number (1) is correct.
Example 4: H2O has a net dipole moment, while BeF2 has zero dipole moment, because:
1)H2O molecule is linear while BeF2 is bent.
2) The BeF2 molecule is linear while H2O is bent.
3)Fluorine is more electronegative than oxygen.
4)Be is more electronegative than oxygen.
Solution
As we have learned,
The structure of H2O and BeF2 are:
By seeing the bond angle we can say that BeF2 molecule is linear while H2O is bent. Moreover, a water molecule is sp3 hybridized and BeF2 is sp hybridized.
Hence, option number (2) is correct.
Example 5: Which one of the following pairs of molecules will have permanent dipole moments for both members?
1) SiF4 and NO2
2) NO2 and CO2
3) NO2 and O3
4) SiF4 and CO2
Solution
Both NO2 and O3 are bent molecules with asymmetry. Hence both will exhibit permanent dipole moment.
Hence, the answer is the option(3).
NCERT Chemistry Notes :
Summary
The dipole moment points from the positive to the negative charge and is quantified by the product of the charge difference and the distance between the charges, expressed in Debye units (D). The presence and magnitude of a dipole moment are determined by the electronegativity of atoms and the molecule's geometry. Polar molecules have significant dipole moments, while nonpolar molecules have zero dipole moments. Understanding dipole moments is essential for predicting molecular behavior in electric fields, interactions with other molecules, and solubility in solvents.
Also check-
Frequently Asked Questions (FAQs)
A dipole moment occurs when the electronegativity of two atoms engaged in a bond is different. The bond's dipole moment and polarity increase as the electronegativity gap between the two atoms grows.
The dipole moment (µ) is the result of multiplying the magnitude of the charge Q by the distance r between the charges to get the net molecular polarity at each end of the molecular dipole. The partition of charges in a molecule is represented by dipolar moments. As a result, this article briefly discusses the definition and formula of dipole moments.
H2O > NH3 > NF3 > BeF2 = CH4
i.e., the dipole moment of the compounds is H2O = 1084D, NH3 = 1.49D,
NF3 =0.24D, BeF2 = CH4 = 0
Permanent Dipole Moments arise when the electronegativity of two atoms in a molecule differs significantly. The atom with the higher electronegativity will attract more electrons, resulting in a partial negative charge surrounding it and a partial positive charge around the atom with the lower electronegativity. Molecules possessing persistent dipole moments, as opposed to dipole moments generated by other molecules, are simply polar molecules. They're significant because a molecule's polarity influences many of its characteristics and interactions with other molecules.
Despite having three polar B—F bonds, BF3dipole moment is zero. Because BF3 has sp2 hybridisation and a regular trigonal planal shape, this is the case. As a result, the individual dipole moments of polar bonds cancel out, resulting in a zero overall dipole moment.
