Pinacol Pinacolone Rearrangement Process with FAQs
Pinacol pinacolone rearrangement – Introduction
Before answering the question “what is pinacol pinacolone rearrangement?” We first need to understand the meaning of pinacol and pinacolone.
Pinacol – pinacol is a vicinal diol which has hydroxyl group (-OH) on vicinal carbon. It is a solid organic compound; white in color. It is produced when acetone undergoes pinacol coupling reaction. Because it is a vicinal diol, pinacol undergoes rearrangement when heated with sulfuric acid to form pinacolone. Pinacol reacts with boron and boron trichloride to give useful synthetic intermediates like pinacolborane, pinacol chloroborane and bis(pinacolato)diboron. Pinacol iupac name is 2,3-dimethylbutane-2,3-diol.
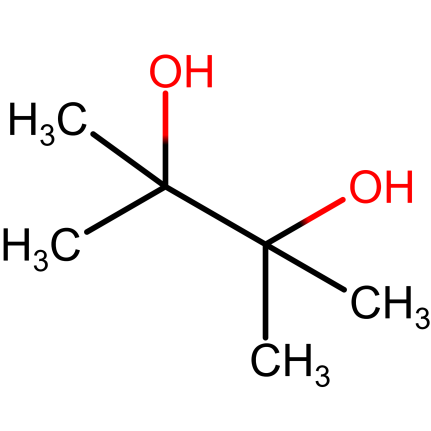
Figure.1. Pinacol structure
Pinacolone – pinacolone is a ketone which is a colorless liquid with a gentle peppermint-like or camphor-like smell. The IUPAC name of pinacolone is 3, 3-dimethyl-2-butanone. Pinacolone is an unsymmetrical ketone having an alpha methyl group which can participate in condensation reactions. Being a ketone, pinacolone also has a carbonyl carbon that can undergo nucleophilic addition reactions like hydrogenation etc. Pinacol undergoes protonation to form pinacolone. In industries, pinacolone is produced by hydrolyzing 4, 4, 5-trimethyl-1, 3-dioxane which is formed in Prins reaction. When pivalic acid and acetic acid (or acetone) are ketonized in presence of metal oxide catalyst they form pinacolone. Pinacolone is also formed by converting 2,3-dimethyl-2-butene. 2-methyl-2-butanol when reacted with C5 alcohols produce pinacolone.
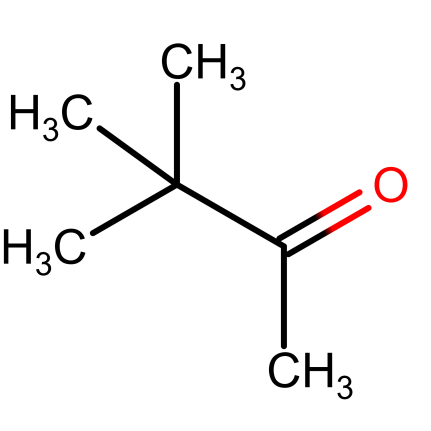
Figure.2. Pinacolone structure
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Pinacol pinacolone rearrangement (or pinacol rearrangement)
In 1860, pinacol pinacolone reaction was first illustrated by Wilhelm Rudolph Fittig. The pinacol–pinacolone rearrangement involves conversion of a 1, 2-diol to a carbonyl compound via 1, 2-shift under acidic conditions. This reaction is called pinacol pinacolone rearrangement because 1,2-diol is called pinacol and the converted carbonyl group is called pinacolone (which is a ketone). In 1, 2-shift, a functional group present on the carbon atom or C1 is relocated on the next carbon or C2.
Pinacol pinacolone reaction
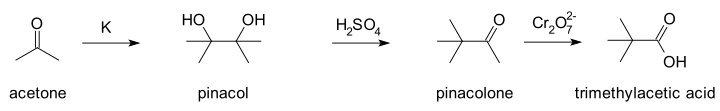
Pinacol pinacolone rearrangement mechanism
In pinacol pinacolone rearrangement, the reaction starts with protonation of one of the alcohols of vicinal diol or 1,2-diol or pinacol. This generates an oxonium ion which has a positive charge on a highly electronegative oxygen atom. Due to destabilized oxonium ion removal of water takes place from the carbon.
This process of removal of water generates carbocation as an intermediate. Carbon with positive charge is called carbocation. This carbocation undergoes a 1,2-methyl shift. 1,2-methyl shift involves relocation of methyl groups from one carbon to another. Due to this methyl shift, a new carbocation is formed. This newly formed carbocation is resonance stabilized. Since an oxygen atom has lone pairs of electrons, a pair of electrons is migrated on the electron deficient carbocation leading to the formation of pi bond between carbon and oxygen. This newly formed carbonyl compound or ketone is called pinacolone.
Pinacol pinacolone reaction is a regioselective reaction that means in pinacol pinacolone rearrangement, the major or single product is formed due to rearrangement of carbocation which is more stable.
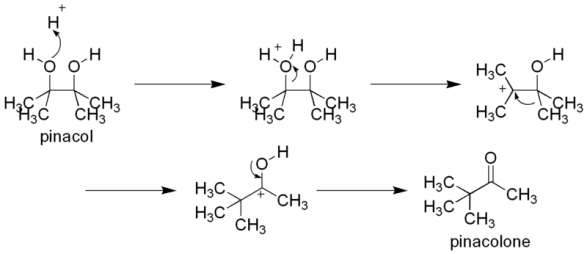
One of the important characteristics of pinacol pinacolone rearrangement is that the configuration of the migration group is retained or remains unchanged.
Migratory aptitude in pinacol pinacolone rearrangement
This process is not limited to 1,2-methyl shifts. A carbon can have different types of groups attached to it. Some of the group may rearrange more readily than others. Depending on the reaction conditions and on the nature of the substrate, it could be deduced which group might migrate.
Generally, the order of migration of groups can be given as:
H > aryl > alkyl
If hydrogen is present as a migratory group, aldehydes can also be produced by undergoing 1,2-shift.
As observed, the more nucleophilic a group is, the more readily it will migrate. Order of migration in aryl groups can be given as:
p-anisyl > p-tolyl > phenyl > p –chlorophenyl
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
Application of pinacol pinacolone rearrangement
There are various synthesis processes of pharmaceutical in which pinacolone; a product of pinacol pinacolone rearrangement is used:
- widely used in large amounts for synthesis of pesticides, fungicides, and herbicides.
- used in retrosynthetic analysis of vibunazole.
- used in production of drugs like pinacidil and naminidil.
- used in stritipenol; a drug used as an epilepsy medication.
- used in synthesis of Diethylstilbestrol pinacolone; a nonsteroidal estrogen medication.
- used in triadimefon; a fungicide used to treat fungal disease.
- used in paclobutrazol; a triazole fungicide and plant growth retardant.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: