Swarts Reaction - Mechanism, Application, Examples, FAQs
Have you ever thought about how scientists replace one type of atom in a molecule with another to create new compounds? What happens when the halogen atom in an alkyl halide is exchanged for a fluorine atom? The answer of these questions is Swarts reaction. It is a process of converting alkyl chloride or alkyl bromide to form alkyl fluoride. It is an organic reaction. The reaction got its name after Frederic Jean Edmond Swarts who reported and explained this reaction for the first time in 1892.
This Story also Contains
- Swart's Reaction
- Swarts Reaction Mechanism
- Applications of Swarts Reaction
- Finkelstein Reaction
- Some Solved Examples
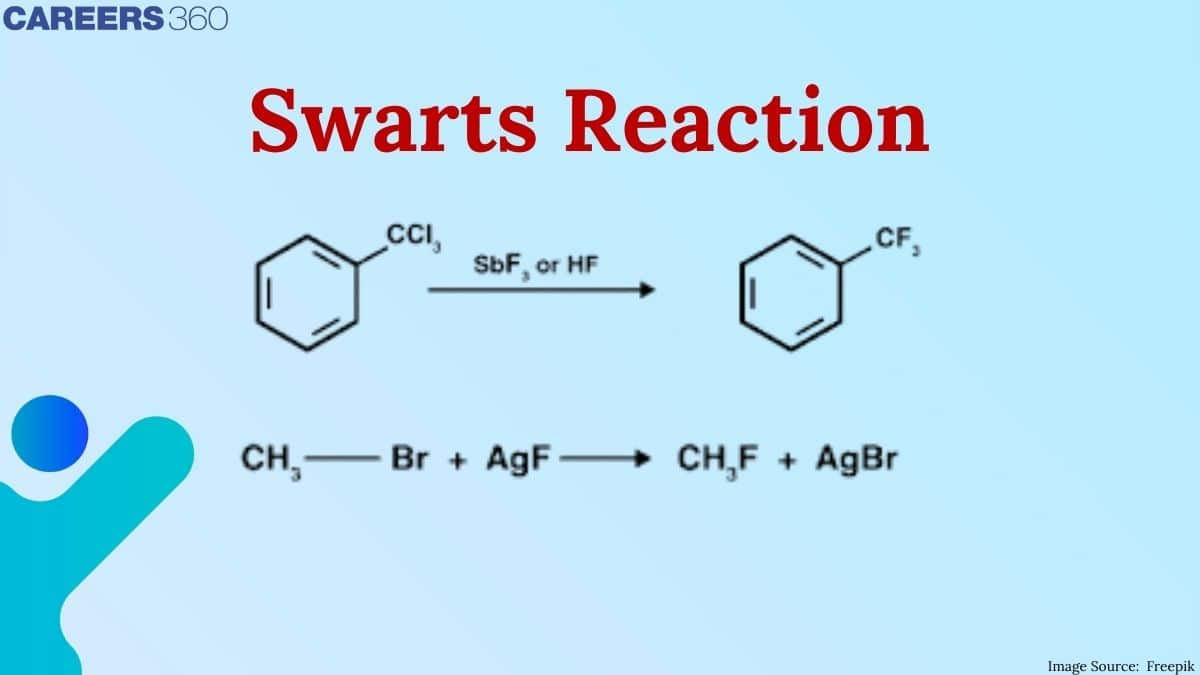
In this article, we cover the swarts reaction which is the topic of class 12 of chapter haloalkanes and haloarenes. Which is very important for boards and JEE Mains Exam, NEET Exam, and many others.
Swart's Reaction
The best method for the preparation of alkyl fluorides is the Swarts reaction. This reaction is done by heating Alkyl chloride or alkyl bromide in the presence of heavy metal fluorides. We can use mercurous fluoride or silver fluoride in the form of heavy metal fluorides. If we use light metal fluorides, then the reaction will continue in the forward direction, but the product formed will be much less. This is simply a fluorination reaction.
Example of Swarts reaction
1. $\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{AgF} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{~F}+\mathrm{AgCl}$
2. $\mathrm{CH}_3 \mathrm{Br}+\mathrm{AgF} \rightarrow \mathrm{CH}_3 \mathrm{~F}+\mathrm{AgBr}$ ( in the presence of fluoride of heavy metals and swarts reagent)
Swarts reaction is the best reaction for the formation of alkyl fluorides with the help of Swarts reagent i..e... $\left(\mathrm{SbF}_3+\mathrm{Cl}_2\right)$ and heavy metals fluorides such as $\mathrm{AgF}, \mathrm{F}_2 \mathrm{Hg}_2, \mathrm{CoF}_2$, or $\mathrm{SbF}_3$.
In general, if we have to prepare metal fluorides we use swarts reaction or swarts fluorination reaction.
What happens in this reaction?
There will be a small answer based on a concept ( higher electronegative elements replace lower electronegative elements).
According to this concept, we all can well notice that chlorine is generally replaced by fluorine in this reaction. In the presence of antimony trifluoride with antimony salts swart reaction will smoothly move forward due to the +5 oxidation state of antimony salt.
Swarts Reaction Mechanism
This reaction is based on bond breakdown and formation of new bonds i.e. the bond between metal and fluorine will break down and a new bond will be formed between carbon and fluorine. This is generally a displacement reaction in which the displaced bromine or chlorine atom forms a bond with the metal. For the swarts reaction, the reagent we used was a mixture of antimony trifluoride and chlorine. The fluoride formed after swarts reaction will have a low boiling point as compared to the corresponding fluoride according to the role of swarts.
We can also use chlorinated hydrocarbons with metallic fluorides to get hydrocarbons containing both chlorine and fluorine.
We use, Swarts reaction or Swarts fluorination for displacement of chlorine or bromine containing alkyl group with fluorine, and forms alkyl fluoride itself.
A variant of the swart reaction is very common in the formation of freons. The main activity of this variation is that fluorination is executed in the presence of hydrogen fluoride and antimony salt in which the oxidation state of antimony salt is +3 & +5.
.png)
Applications of Swarts Reaction
- For the production or formation of alkyl fluoride.
- Used in the formation of freons.
- Swarts Reaction is used for chemical analysis.
Also read -
Finkelstein Reaction
This is also a halogen exchange method and its post-reaction product is alkyl iodide.
Finkelstein reaction occurs through Substitution Nucleophilic Bimolecular reaction i.e. Sn2 reaction. This is also a type of organic reaction. In this reaction, primary alkyl halide or pseudohalide reacts with alkali metal halide to form alkyl iodide ( this reaction is based on halogen exchange ).
Commonly used reagents in this reaction are:-
- Sodium iodide – acts as a nucleophile in this reaction
- Ethyl chloride – an alkyl halide to complete this reaction.
- The product is Ethyl iodide i..e.. formed due to the exchange between the iodine and chlorine groups. So it is called the halogen exchange process.
Examples of Finkelstein reactions are:-
- $\mathrm{CH}_3 \mathrm{Br}+\mathrm{NaI} \rightarrow \mathrm{CH}_3 \mathrm{I}+\mathrm{NaBr}$ (reaction will happen in the presence of acetone)
- $\mathrm{CH}_3 \mathrm{Cl}+\mathrm{NaI} \rightarrow \mathrm{CH}_3 \mathrm{I}+\mathrm{NaCl}$ (reaction will happen in the presence of acetone)
- $\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}+\mathrm{KI} \rightarrow \mathrm{C}_2 \mathrm{H}_5 \mathrm{I}+\mathrm{KCl}$ (this reaction will happen in the presence of acetone)
Related Topics,
Some Solved Examples
Question 1. Which reagents are used in the swarts reaction?
Ans: The reagents used in swarts reactions are chlorine or many times antimony pentachloride also used with antimony trifluoride. The combination of both these reagents (..e..chlorine and antimony trifluoride) is known as a swarts reagent.
Swarts reagent $=\mathrm{SbF}_3+\mathrm{Cl}_2$
Question 2. What is the product of Finkelstein's reaction?
Ans: Finkelstein's reaction is named after a German chemist. i.e. Hans Finkelstein.
The product of the Finkelstein reaction is alkyl iodide which is formed due to halogen exchange. Finkelstein reaction follows the sn2 ( Nucleophilic Substitution, Second Order ) pathway to form the product.
Question 3. Similarity between swarts reaction and finkelstein reaction?
Ans: These both given reaction is halogen exchange reaction. Both of these reactions follow the sn2 (second-order Nucleophilic substitution) pathway to form a product. In the Swarts reaction product is alkyl fluoride and in the Finkelstein reaction product will be alkyl iodide.
Question 4. Difference between swarts reaction and finkelstein reaction?
Ans: In swarts reaction, reagents are heavy metal fluorides that can easily fluorinate alkyl components. This reaction is used to form alkyl fluoride with good yield. The reagents of the Swarts reaction are SbF3 + Cl2 Whereas in the Finkelstein reaction, we get alkyl iodide, not fluoride.
The Reactant of this reaction is sodium iodide in acetone ( NaI + C3H6O ) Both of these are organic reactions and widely play an important role in many organic reactions, analysis, etc.
Question 5. Best method for the preparation of alkyl fluoride?
Ans: Swarts reaction is the best method to form alkyl fluoride by substituting chlorine or bromine. It is a halogen exchange method that completes in the presence of swarts reagent i..e. $\left(\mathrm{SbF}_3+\mathrm{Cl}_2\right)$
Here
- SbF3 = antimony trifluoride
- Cl2 = molecular chlorine
Question 6. What are freons?
Ans: It is fluorinated aliphatic organic compounds, which are very useful in commerce and industries.
Constituents of freons:- fluorine + carbon ( must contain these two elements)
Freons can also contain elements like hydrogen, chlorine, or bromine.
There are many types of freons including chlorofluorocarbons ( CFCs), hydrochlorofluorocarbons ( HCFCs), etc.
Freons have been used as refrigerants since the 1930s because of some special properties including colorlessness, odorlessness, inflammability, and less corrosive and toxic gas. Freons can occur in two stages depending on environmental temperature i..e.. liquid or gases. They also have low boiling points, low viscosity, and low surface tension that helps them to be a refrigerant of great value.
Examples of freons:-
- Dichlorodifluoromethane (R-12)
- Tetrafluoroethane (R-134a)
Question 7. How to convert methyl chloride to methyl fluoride?
Ans: The answer will be through Swarts reaction we can convert it.
$\mathrm{CH}_3-\mathrm{Cl}+\mathrm{SbF}_5 \rightarrow \mathrm{CH}_3-\mathrm{F}$
When we react methyl chloride with swarts reagents like $\mathrm{F}_2 \mathrm{Hg}_2, \mathrm{SbF}_5$ then methyl chloride undergoes a halogen exchange reaction in which methyl chloride loses its chlorine and gains fluorine.
And by this process, methyl chloride will be converted to methyl fluoride.
Question 8. Is F2Hg2 a reagent of the swarts reaction?
Ans: Yes, it is a very good fluorinating agent and also a reagent for swarts reaction.
$\mathrm{F}_2 \mathrm{Hg}_2$ – Compound name is “ Mercury(I) fluoride “
Question 9. What is Swarts reaction?
Ans: It is a process by which chlorine-containing organic compounds are changed to fluorides through a halogen exchange process with the help of swarts reagent i..e.. in the presence of antimony trioxide and chlorine. This reaction follows SN2 i..e. Nucleophilic substitution pathway.
Also, check-