P-block Elements - Notes, Topics, Formula, Books, FAQs
Have you ever thought about why does nitrogen form a triple bond while oxygen prefers a double bond? How can carbon create millions of compounds, yet noble gases hardly react at all? You will get all these answers after studying p-block elements. P-block consists of groups 13 to 18 in the periodic table having the general electronic configuration as ns2np1-6. This special group has metals, non-metals and metalloids as well as different elements.
This Story also Contains
- Important Topics Of p-block Elements
- Overview Of The Chapter
- How to Master p-block Elements: A Strategic Preparation Guide
- Previous Year Questions Of P-Block Elements
- Prescribed Books
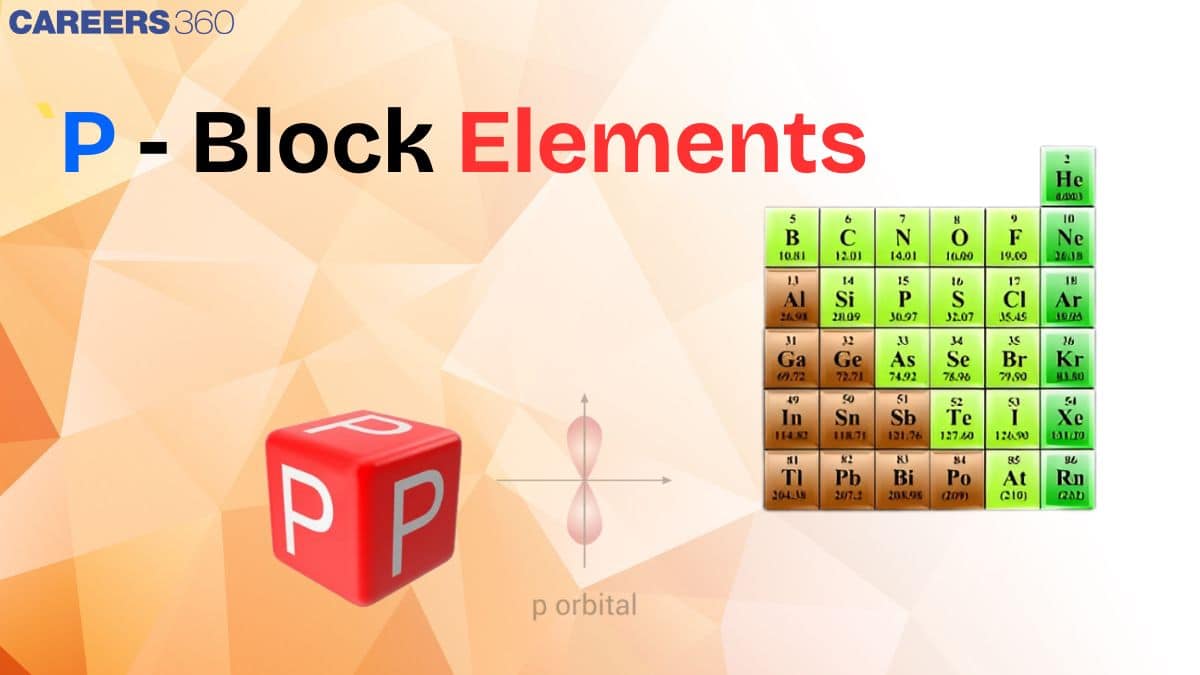
P-block block contains halogens, noble gases, carbon, nitrogen, and oxygen as their essential elements. The elements in this block form covalent compounds and exist in different oxidation states. The chemical behaviour is strongly affected by the inert pair effect, ionization energy, and electronegativity of the specific elements. The mentioned properties thus produce a broad spectrum of chemical and physical characteristics. Emphasis on the p-block elements is important in this era because of their diversity in characteristics and critical uses, which proves to be the basis for several chemical, industrial, and biological processes.
Important Topics Of p-block Elements
Physical Properties of the Boron Family
The boron family, Group 13 elements (B, Al, Ga, In, Tl), shows a range of physical properties. Boron is a non-metal, while others are metals. Their melting and boiling points vary, with boron having the highest melting point due to its covalent structure. The atomic and ionic sizes increase down the group, and density also increases except for irregularities like gallium.
Anomalous Behaviour of Boron
Anomalous behaviour of Boron means it deviates from the rest of its group due to its small size, high ionization energy, and absence of d-orbitals. Unlike metals in its group, boron forms covalent bonds and shows non-metallic properties. It also forms compounds like borates and boric acid, which are unique to it.
Chemical Properties of the Boron Family
The Boron family reacts with acids, bases, and halogens. Boron forms covalent compounds, while others can form ionic ones. For example, aluminium reacts with acids and bases, showing its amphoteric nature. The tendency to form stable +3 oxidation states decreases down the group due to the inert pair effect.
Diborane
Diborane (B2H6) is a covalent hydride of boron with a unique structure featuring three-center two-electron bonds (banana bonds). Diborane is a colorless, flammable gas used as a reducing agent and in organic synthesis. It reacts with water, releasing hydrogen and forming boric acid.
Orthoboric Acid
Orthoboric acid (H3BO3) is a weak monobasic acid that forms by dissolving borax in water. It is used as an antiseptic and in glass manufacturing. It has a layered structure with hydrogen bonding and acts as a Lewis acid by accepting hydroxide ions.
Borax Formula
Borax is a key boron compound has the chemical formula Na2B4O7⋅10H2O. Borax is used in glass and ceramics production, detergents, and as a flux in metallurgy. On heating, borax gives a bead test, forming a characteristic glassy bead of boric oxide.
Diagonal Relationship of Boron and Silicon
Boron and silicon exhibit a diagonal relationship due to their similar electronegativity and ionic sizes. Both form covalent compounds, hydrides, and oxides with acidic properties. They also share structural similarities in borates and silicates.
Group 14 Elements (Carbon Family)
Group 14 elements (Carbon family) exhibit trends in metallic and non-metallic behaviour. Carbon and silicon are non-metals, while others are metals. The +4 oxidation state is more stable in lighter elements, whereas the +2 state becomes predominant in heavier ones due to the inert pair effect.
Allotropes of Carbon
Allotropes of Carbon are diamond, graphite, and fullerenes. Diamond has a tetrahedral structure, making it the hardest natural substance, while graphite has a layered structure with delocalized electrons, making it a good conductor of electricity.
Carbon Monoxide and Carbon Dioxide
Carbon monoxide (CO) is a colorless, toxic gas with strong reducing properties. Carbon dioxide (CO2) is a non-toxic gas, essential for photosynthesis. Both play crucial roles in biological and industrial processes.
Silicates
Silicates are compounds containing silicon-oxygen tetrahedra. They form the majority of Earth's crust and are found in minerals like quartz and feldspar. Their structures vary from simple to complex, influencing their properties and uses.
Silicon Dioxide
Silicon dioxide (SiO2) is also known as silica, is a major component of sand. It forms a network structure and is used in glassmaking, electronics, and construction. It is chemically inert and resistant to high temperatures.
Silicones
Silicones are synthetic polymers with silicon-oxygen backbones and organic side groups. They are used in lubricants, sealants, and medical implants due to their flexibility, water resistance, and thermal stability.
Group 15 Elements (Nitrogen Family)
Group 15 elements (Nitrogen family) show trends in non-metallic to metallic behaviour down the group. Nitrogen forms diatomic molecules with a triple bond, while heavier elements exhibit metallic and amphoteric properties.
Dinitrogen - Preparation, Properties, and Uses
Dinitrogen is prepared by fractional distillation of liquid air or by thermal decomposition of ammonium dichromate. Dinitrogen is inert due to the strong triple bond and is used in fertilizers, explosives, and as a refrigerant.
Preparation and Properties of Ammonia
Ammonia is prepared by the Haber process. It is a colorless gas with a pungent odor, soluble in water, and acts as a weak base. Ammonia is widely used in fertilizers and cleaning products.
Oxides of Nitrogen
Nitrogen forms a variety of oxides with diverse oxidation states. Oxides of Nitrogen are important in environmental chemistry as they contribute to pollution and acid rain.
Phosphorus
Phosphorus exists in several allotropes, including white, red, and black phosphorus. White phosphorus is highly reactive and stored under water, while red phosphorus is less reactive and used in matchstick production.
| Also read, |
|
Overview Of The Chapter
P-block elements consist of elements from group 13 to group 18. These elements have various properties and characteristics that are important to learn. These p-block elements are completely different from each other. Some members of this group are metals like aluminum, some are metalloids like silicon and germanium, and rest are non-metals like oxygen, chlorine, etc.
Electronic Configuration
The general electronic configuration of p-block elements is given as follows:
Group 13 elements: ns2 np1
Group 14 elements: ns2 np2
Group 15 elements: ns2 np3
Group 16 elements: ns2 np4
Group 17 elements: ns2 np5
Group 18 elements: ns2 np6
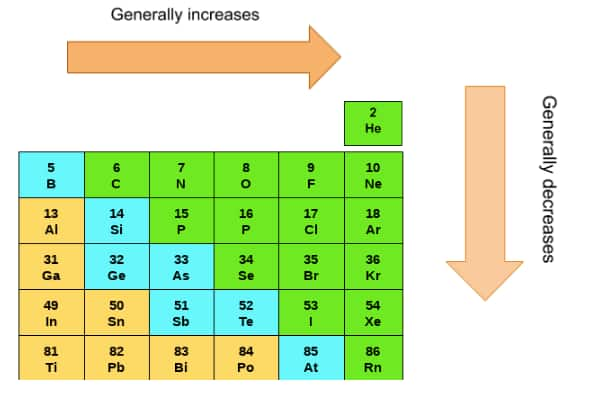
Metallic Character
In the p-block series, the elements are classified into various categories based on their metallic character. The metallic character of the elements increases as we move down the group. There are some elements that are between the metallic and non-metallic characters, known as metalloids. Group 17 elements are purely non-metals known as halogens. Group 18 elements are known as noble gases.
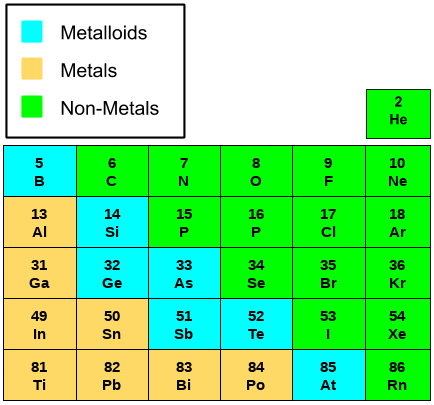
Atomic Radii
As we move down the group, there is always the addition of one more shell; due to this, the radius of the elements always increases in moving down the group. But as we move from left to right, the atomic size decreases. This is because, from left to right in the same period, no extra shell is added,d but the nuclear charge is increased and thus the size is decreased
Ionization Enthalpy
The ionization energy of p-block elements increases from left to right because of the effective nuclear charge on the outer electrons increases. But from top to bottom, this ionization energy decreases as the size of the atom increases, due to which the influence of the nucleus on the electrons decreases, and thus it is easy to remove the electron from the outermost shell.
Electronegativity
The electronegativity of p-block elements increases from left to right as the effective nuclear charge increases. From the bottom, the electronegativity decreases because of increased atomic size and lesser influence of the nucleus.
Melting and Boiling Points
The melting and boiling points of all the p-block elements increase as we move down the groups. This is because of the fact that as we move down the group, the atomic size of elements increases due to which the melting and boiling points increase.
Oxidation States
The p-block elements show variable oxidation states. Generally, the value of their oxidation states is negative but when bonded with more electronegative atoms then they also exhibit positive oxidation states. The maximum oxidation state shown by the atom is equal to the number of valence electrons. The oxidation states of different elements are shown below:
- Boron family: +3
- Carbon family: -4 and +2
- Nitrogen family: -3, +3 and +5
- Oxygen family: -2, +2, +4 and +6
- Halogen family: -1, +1, +3, +5 and +7
- Noble gases: +2, +4, +6 and +8
Magnetic properties
Almost all the elements of the periodic table are diamagnetic in nature. Some elements like iodine, Astatine, Radon, and Polonium are non-magnetic. Only Tin is the element that is paramagnetic.
Color of p-block elements
- Color of group 13 elements:
All the elements are silvery in color but the boron is brown. - Color of group 14 elements:
The colour of carbon is black and silicon and germanium are grey coloured. - Color of group 15 elements:
In this group, all the elements have different colors. Nitrogen is colorless, phosphorus is red and white, arsenic is yellow, antimony is grey and bismuth is silvery white. - Color of group 16 elements:
In this group, oxygen is colorless, sulfur is pale yellow, selenium is red, tellurium is silvery-white and polonium is silvery. - Color of group 17 elements:
All the halogens have different colors. Fluorine is pale yellow, Chlorine is greenish-yellow, bromine is reddish-brown, and iodine is violet-black. - Color of group 18 elements:
All noble gases have different colors. Helium is red, Neon is orange, krypton is purple, Xenon is white, and Radon is colorless.
These elements have various real-life applications that we observe around us on a daily basis. Some of them are mentioned below.
- Aluminum is a very important member of p-block elements. It is in the manufacturing of airplanes.

- Chlorine belongs to group 17 in the periodic table. It is used in swimming pools to sanitize the water and protect the swimmers from waterborne diseases.

How to Master p-block Elements: A Strategic Preparation Guide
-
This chapter is a part of inorganic chemistry. It is completely theory-based and very easy to learn; no need to memorize any formulas.
-
Before reading this chapter, first, you must have the basic knowledge of the chapter - periodic classification of elements.
-
You must also learn why there are some elements like Boron and Carbon show anomalous behavior with respect to other elements in their group.
-
The rest of this complete chapter is very simple, just be regular and be consistent in your practice.
Previous Year Questions Of P-Block Elements
Question 1. The compound that does not produce nitrogen gas by the thermal decomposition is :
1. $\left(\mathrm{NH}_4\right)_2 \mathrm{SO}_4$
2. $B a\left(N_3\right)_2$
3. $\left(\mathrm{NH}_4\right)_2 \mathrm{Cr}_2 \mathrm{O}_7$
4. $\mathrm{NH}_4 \mathrm{NO}_2$
Answer 1. Laboratory method of nitrogen preparation -
By heating an aqueous solution of NH4Cl and NaNO2
- wherein
$\begin{aligned} & \mathrm{NH}_4 \mathrm{Cl}+\mathrm{NaNO}_2 \xrightarrow{\Delta} \mathrm{~N}_2+2 \mathrm{H}_2 \mathrm{O}+\mathrm{NaCl} \\ & \mathrm{NH}_4 \mathrm{NO}_2 \xrightarrow{\Delta} \mathrm{~N}_2 \uparrow+2 \mathrm{H}_2 \mathrm{O} \\ & \left(\mathrm{NH}_4\right)_2 \mathrm{Cr}_2 \mathrm{O}_7 \xrightarrow{\Delta} \mathrm{~N}_2 \uparrow+\mathrm{Cr}_2 \mathrm{O}_3+\mathrm{H}_2 \mathrm{O} \\ & \mathrm{Ba}\left(\mathrm{N}_3\right)_2 \xrightarrow{\Delta} \mathrm{~N}_2 \uparrow+\mathrm{Ba}_{(\mathrm{s})}\end{aligned}$
Hence , the answer is the option (1).
Question 2. In graphite and diamond, the percentage of p-characters of the hybrid orbitals in hybridisation are respectively :
1. 32 and 25
2.33 and 25
3. 50 and 75
4. 67 and 75
Answer 2.
As we have learned,
Graphite has $\mathrm{sp}^2$ hybridization
$\% \mathrm{p}-$ character $=\frac{2}{3} \times 100=67 \%$
Diamond has $\mathrm{sp}^3$ hybridisation
$\% \mathrm{p}-$ character $=\frac{3}{4} \times 100=75 \%$
Hence, the answer is the option (4).
Practice More Questions From The Link Given Below
For more questions to practice, the following MCQs will help inthe preparation for competitive examinations
Prescribed Books
First, you must finish the class XI and XII NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously give mock tests for the depth of knowledge. Our platform will help you to provide a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Also read,
Frequently Asked Questions (FAQs)
P-block elements are those in which the last electron enters the outermost p-orbital. They include elements from Group 13 to Group 18 of the periodic table. They are called "p-block" because their valence electrons occupy the p-subshell.
The first element of each group (e.g., N, O, F, C, B) shows anomalous behaviour compared to other elements in its group due to:
- Small Size: This leads to high ionization enthalpy and high electronegativity.
- Absence of d-orbitals: This limits their covalency to a maximum of four (e.g., Nitrogen cannot form NCl5, unlike Phosphorus which can form PCl5). It also prevents them from expanding their octet.
- Ability to form pπ-pπ multiple bonds: Especially with itself (e.g., N≡N, O=O) and with other small, highly electronegative elements (e.g., C=O, C≡N).
- They show a wide variation in properties, from non-metals (like carbon, nitrogen, oxygen) to metalloids (like silicon, germanium, arsenic) to metals (like aluminium, tin, lead, bismuth).
- Their valency and oxidation states vary significantly.
- They tend to form covalent compounds, especially the non-metals, but also ionic compounds with highly electropositive metals.
- Their general electronic configuration is ns²np¹⁻⁶.
- Oxides of non-metals are generally acidic, while those of metals are basic or amphoteric.
The first elements (e.g., B, C, N, O, F) exhibit unique properties due to their small size, high electronegativity, and absence of d-orbitals. Examples include carbon’s catenation and nitrogen’s diatomic (N≡N) stability.
Metalloids, such as silicon and germanium, possess properties that are intermediate between metals and nonmetals. They are vital in the semiconductor industry, which is essential for electronics, solar cells, and various other applications.
The inert pair effect refers to the reluctance of the ns² electrons (in heavier p-block elements like Pb, Tl, and Bi) to participate in bonding, making lower oxidation states (e.g., Pb²⁺, Tl⁺) more stable than higher ones (e.g., Pb⁴⁺, Tl³⁺).
Halogens (F₂, Cl₂): Toxic and corrosive; require ventilation.
Arsenic (As), Lead (Pb): Carcinogenic; need proper disposal.
Boron Hydrides (e.g., B₂H₆): Highly flammable.
Group 13 (Boron group): Aluminum (lightweight alloys), Boron (semiconductors, borosilicate glass).
Group 14 (Carbon group): Silicon (electronics), Carbon (organic chemistry, nanomaterials).
Group 15 (Nitrogen group): Nitrogen (fertilizers), Phosphorus (matches, detergents).
Group 16 (Chalcogens): Oxygen (medical, combustion), Sulfur (sulfuric acid production).
Group 17 (Halogens): Chlorine (disinfectants), Fluorine (Teflon, refrigerants).
- Group 18 (Noble gases): Argon (welding), Neon (lighting).