Calorimeter - Definition, Uses, Types, Application, Diagram, FAQs
Calorimetry is the science of measuring the amount of heat released or absorbed during chemical reactions, physical changes, or when substances are heated or cooled. Based on the principle of conservation of energy, calorimetry ensures that the total energy in a closed system remains constant. Heat is transferred between objects or substances until thermal equilibrium is reached, where the system's total energy remains balanced.
This Story also Contains
- Definition of Calorimetry
- Principle of Calorimeter
- Calorimetry Uses
- Solved Examples Based on the Calorimetry Principle
- Summary

In everyday life, calorimetry plays a vital role. For example, when you heat water for tea, the stove transfers heat to the water, gradually increasing its temperature until it boils. Similarly, when you sweat, your body releases heat to the surrounding air, cooling you down—illustrating how energy moves and changes in common scenarios. This principle is also crucial in various industries, from food production to designing cooling systems for electronics.
Definition of Calorimetry
The study of the measurement of the changes that occur in the state variable of anybody to find the amount of heat transfer that happens along with state transfer is defined as calorimetry. For example, finding the change of state variable during any phase transitions or other kinds of physical changes. The calorimeter is the instrument used in the study of calorimetry.
Principle of Calorimeter
Consider any two different bodies such as one solid and one liquid in different temperatures. Place the two different bodies such that both are in physical constant. The observations show that the heat transformations occur from the higher temperature body to the lower temperature body. This transformation occurs until both bodies attain thermal equilibrium. The heat is released by the higher-temperature body and the heat is absorbed by the lower-temperature body. Thus the energy is conserved in this process. The calorimeter also works with the same principle. The main principle of the calorimeter is the law of conservation of energy. The heat loss of one body is compensated by the heat gained by another body.
The formula of the Calorimeter with which it works:
From the conservation of the energy, Heat loss = heat gain
q=mcΔt
Where q denotes the measure of the transfer of heat
m denotes the body mass
c denotes the specific heat of the object
Δtdenotes the temperature change
Calorimeter - Explanation in Detail
The physical instrument or device which is used in the measurements of heat-related stuff (that is mainly used in the study of calorimetry). The construction of the calorimeter contains vessels made up of good conductors like metals. The composition of metallic vessels contains copper and aluminium in major. The contents of the metallic vessel are facilitated for stirring with the help of a stirrer. The heat loss in the stirrer is reduced with the help of an insulated jacket around the stirrer. The thermometer is allowed to be inserted through a small opening which is used to measure the thermal change that occurs inside the calorimeter.
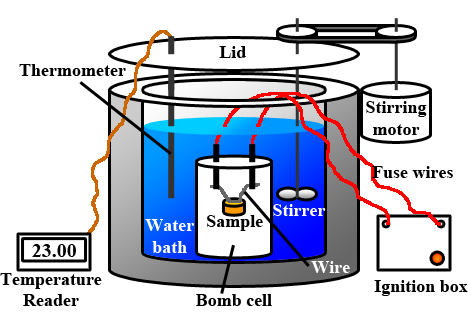
In the inner part of the calorimeter, some sample of fuel is used to burn. The metallic vessel contains water, when the fuel inside is burnt, the water gets heated. The heat loss of the fuel is converted into the heat gained by the water. That is, energy is conserved. To increase the accuracy of results, heat loss can be terminated by the insulation of the calorimeter instrument from the environmental conditions. The heat change of the water is measured by using the inserted thermometer. The readings are used to find the heat capacity of the water and the amount of energy conserved inside the fuel.
Calorimetry Uses
A detailed study in the thermodynamic analysis of the substance and environment is required to answer the relationship between the particles, their structure and other properties of that particle.
Calorimeter Types
There are different types of Calorimeters used in practice. Some of the calorimeters are listed below: they are;
- Adiabatic calorimeters
- Constant pressure calorimeter
- Reaction calorimeters
- Differential scanning calorimeter
- Bomb calorimeters
Recommended Topic Video
Solved Examples Based on the Calorimetry Principle
Example 1: Principle of calorimetry is based on
1) conservation of mass
2) conservation of energy
3) conservation of momentum
4) both 1 and 2
Solution:
Calorimetry
Heat lost = Heat gained
wherein
Represents the law of conservation of energy
Hence, the answer is the option 2.
Example 2: An unknown metal of mass 192 g heated to a temperature of 1000 C was immersed into a brass calorimeter of mass 128 g containing 240 g of water at a temperature of 8.40C. Calculate the specific heat (in J Kg-1 K-1) of the unknown metal if the water temperature stabilises at 21.50 C. (The specific heat of brass is 394 J Kg-1 K-1)
1) 458
2) 1232
3) 654
4) 916
Solution:
From the law of conservation of energy
Heat lost = Heat gained
So
192×s(100−21.5)=128×394(21.5−8.4)+240×4200(21.5−8.4)s=916Jkg−1 K−1
Hence, the answer is the option (4).
Example 3: A liquid of mass m and specific heat C is heated to a temperature "2T". Another liquid of mass m/2 and specific heat 2 C is heated to a temperature T . If these two liquids are mixed the resulting temperature of the mixture is
1) 2T/ 3
2) 8T/5
3) 3T/5
4) 3T/2
Solution:
The mixture of two substances
θmix=m1c1θ1+m2c2θ2m1c1+m2c2
wherein
θmix = The temperature of the mixture at equilibrium.
Tmix=m1C1T1+m2C2T2m1C1+m2C2=mc⋅2T+m2⋅2C⋅Tmc+m/2⋅2C=3mCT2mC=3T2
Hence, the answer is the option (4).
Example 4: Ice at −20∘C is added to 50 g of water at 40∘C. When the temperature of the mixture reaches 0∘C, it is found that 20 g of ice is still unmelted. The amount of ice (in gm) added to the water was close to :
(Specific heat of water =4.2 J/g/∘, Specific heat of ice =2.1 J/g/∘C, Heat of fusion of water at 0∘C=334 J/g )
1) 100
2) 50
3) 40
4) 60
Solution:
A mixture of two substances
θmix=m1c1θ1+m2c2θ2m1c1+m2c2 wherein θmix= The temperature of the mixture is at equilibrium. Say the amount of ice =mgm heat taken by ice = heat given by water 20×2.1×m+(m−20)334=50×4.2×40⇒m=40.1≈40
Hence, the answer is 40.
Example 5:5 g of water at 10∘C is mixed with 5 g of water at 40∘C, then final temperature (in celsius) of the mixture is
1) 20
2) 25
3) 30
4) 35
Solution:
If m1=m2 and c1=c2θmix=θ1+θ22θ=θ1+θ22=40+102=25∘C
Hence, the answer is the option (2).
Summary
Calorimetry is the study of heat transfer during physical changes or chemical reactions, governed by the principle of conservation of energy. A calorimeter measures heat exchange between substances, such as the heat lost by a hotter object being equal to the heat gained by a cooler one. This principle has applications in various fields, from everyday activities like boiling water to complex scientific and industrial processes.
Frequently Asked Questions (FAQs)
The concept of calorimetry is used in the measurement of the change in thermal condition of anybody. The study of the measurement of the changes that occur in the state variable of anybody to find the amount of heat transfer that happens along with state transfer is defined as calorimetry. A calorimeter is used to measure and study the calorimetry.
The construction of the calorimeter contains vessels made up of good conductors like metals. The composition of metallic vessels contains copper and aluminium in major. The contents of the metallic vessel are facilitated for stirring with the help of a stirrer. The heat loss in the stirrer is reduced with the help of an insulated jacket around the stirrer. The thermometer is allowed to be inserted through a small opening which is used to measure the thermal change that occurs inside the calorimeter. Inside the calorimeter, some sample of fuel is used to burn. The metallic vessel contains water, when the fuel inside is burnt, the water gets heated. The heat loss of the fuel is converted into the heat gained by the water. That is, energy is conserved. To increase the accuracy of results, heat loss can be terminated by the insulation of the calorimeter instrument from the environmental conditions. The heat change of the water is measured by using the inserted thermometer. The readings are used to find the heat capacity of the water and the amount of energy conserved inside the fuel.
The different types of calorimeters are
Adiabatic calorimeters
Constant pressure calorimeter
Reaction calorimeters
Differential scanning calorimeter
Bomb calorimeters
The main principle of the calorimeter is the law of conservation of energy. the heat transformations occur from the higher temperature body to the lower temperature body. This transformation occurs until both bodies attain thermal equilibrium. The heat is released by the higher temperature body and the heat is absorbed by the lower-temperature body.
Let us discuss the application of calorimeter or The real-life application of calorimetry can be seen in different fields of industries and laboratories. Some examples of the practical calorimeters and their uses are listed below:
Differential scanning calorimeters are used to find the change in any product's formula and its effects
A reaction calorimeter is used to measure the heat generated by the sensors in reactors.
A constant pressure calorimeter helps us to measure the enthalpy change in both physical and chemical methods.
The adiabatic calorimeter is used to evaluate the runtime reactions.
Bomb calorimeters are widely used in different kinds of fields. Some of the fields are analysis of waste products, manufacture of cement, analysis of fuel like coal and explosives, food and nutrition, research in animal feed and so on. For example, the Oxygen bomb calorimeter has its usage in the field of food testing by calculating the amount of heat in food which further helps to determine the calorie of the food.