Electric Charge - Definition, Properties, Formula, Types, Unit, FAQs
Electric charge is a basic physics concept helping to explain the phenomena of attraction and repulsion between objects. It is a property of the matter that causes electric and magnetic effects. Electric charges can be positive or negative in nature. When an object gains or loses electrons, it becomes charged. For example, when you rub a balloon on your hair or feel a small shock after touching a door, it happens because of the movement of electric charges. Charging of an object can be done mainly by three methods: friction, conduction, and induction. The study of electric charge falls under Electrostatics, an important topic in Class 12 Physics, whereby many natural and everyday electrical phenomena are explained.
This Story also Contains
- What is the Electric Charge?
- Types of Charge:
- Properties of charge:
- Coloumb's Law
- Methods of Charging
- Solved Examples Based on Electric Charge
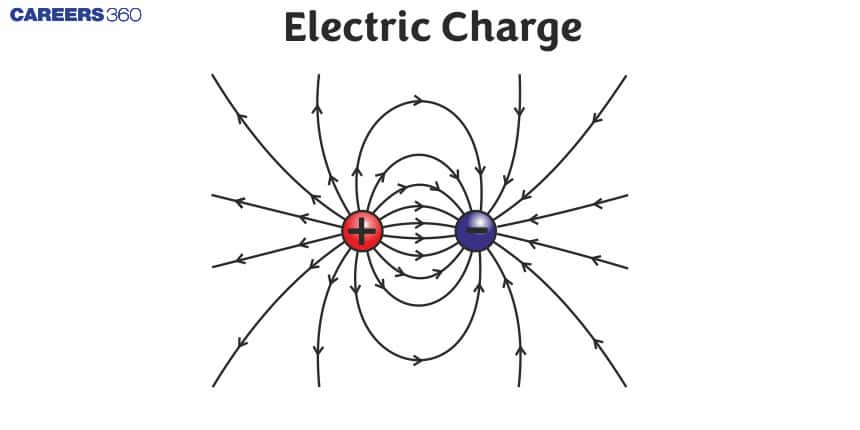
What is the Electric Charge?
In the class 12 Physics electric charge is the important topic and numerical questions and its types are often asked in the exams. Let's discuss the Electric charges and fields in detail.
Electric Charge Definition
Electric charge is a basic property of matter that causes it to experience a force when placed in an electric or magnetic field. It can be positive or negative, just like the charges on protons and electrons. Objects with the same type of charge repel each other, while those with opposite charges attract.
Students are confused whether Charge is a scalar and vector quantity. Answer to this question is that Charge is a scalar quantity.
S.I. Unit of Electric Charge
The SI unit of electric charge is the coulomb, represented by the symbol "C".
Also read -
Types of Charge:
There are two types of electric charges-positive and negative.
- Protons have a positive charge.
- Electrons have a negative charge.
- Neutrons have no charge and are neutral
Interaction of Charges
Like charges (Positive-Positive or Negative-Negative) repel each other (glass rods rubbed with wool or silk repel each other) and unlike charges ( Positive-Negative) attract each other (glass rod and wool attract each other).
Properties of charge:
-
Additivity: If a system contains n charge $q_1, q_2, q_3 \ldots \ldots q_n$, then the total charge of the system is $q_1+q_2+\ldots \ldots q_n$.
-
Conservation of Charge: The charge can be neither created nor destroyed. When we rub a glass rod with silk there is a transfer of charge and not creation. The total charge of an isolated system is always conserved.
-
Unit of Charge: The unit of electric charge is the coulomb (C).
The charge of a single electron is approximately $-1.6 \times 10^{-19}$ coulombs.
Similarly, a proton has a charge of $+1.6 \times 10^{-19}$ coulombs. -
Quantization: The charge on a body will be some integral multiple of e, where e is the charge of the electron.
$e=1.6 \times 10^{-19} \mathrm{C}$
Coloumb's Law
Force between two points charges is explained by the Coloumb's law.
Coloumb's law states that: "The magnitude of the electrostatic force (F) between two point charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them."
it is represented by:
$
F=k \frac{\left|q_1 \cdot q_2\right|}{r^2}
$
where:
- $F$ is the magnitude of the electrostatic force between the charges,
- $q_1$ and $q_2$ are the magnitudes of the two charges,
- $r$ is the distance between the centers of the two charges,
- $k$ is the electrostatic constant, also known as Coulomb's constant, with a value of approximately $9 \times 10^9 \mathrm{Nm}^2 / \mathrm{C}^2$ in a vacuum.
Methods of Charging
- Charging by Friction – When two neutral objects are rubbed together, electrons are transferred from one object to another. For example, rubbing a balloon on hair makes it charged.
- Charging by Conduction – When a charged object touches a neutral object, electrons move from one to the other, and both objects get the same type of charge.
- Charging by Induction – In this method, a charged object is brought near (but not touching) a neutral object. The presence of the charged object causes a separation of charges in the neutral object.
Recommended Topic Video
Solved Examples Based on Electric Charge
Example 1: Charge on $\alpha$-particle is:
1) $4.8 \times 10^{-19} \mathrm{C}$
2) $1.6 \times 10^{-19} \mathrm{C}$
3) $3.2 \times 10^{-19} \mathrm{C}$
4) $6.4 \times 10^{-19} \mathrm{C}$
Solution:
Electric charge
It is the property associated with matter due to which it produces and experiences electrical and magnetic effects.
Alpha particles have a charge of +2 ,
Hence, the charge on an alpha particle is twice the electron charge
$
\begin{aligned}
& =2 \times 1.6 \times 10^{-19} \mathrm{C} \\
& \mathrm{q}=3.2 \times 10^{-19}
\end{aligned}
$
Hence, the answer is the option (3).
Example 2: Which of the following charges is not possible?
1) $1.6 \times 10^{-18} \mathrm{C}$
2) $1.6 \times 10^{-19} \mathrm{C}$
3) $1.6 \times 10^{-20} \mathrm{C}$
4) None of these
Solution:
$1.6 \times 10^{-20} \mathrm{C}$, because this is $\frac{1}{10}$ of electronic charge and hence not an integral multiple.
Hence, the answer is the option (3).
Example 3: When 1019 electrons are removed from a neutral metal plate, the electric charge on it is
1) $ -1.6 C$
2) $+1.6 C$
3) $10^{+19} \mathrm{C}$
4) $10^{-19} \mathrm{C}$
Solution:
Electric charge -The loss of electrons gives a positive charge.
By using
$Q=n e \Rightarrow Q=10^{19} \times 1.6 \times 10^{-19}=+1.6 C$
Hence, the answer is the option (2).
Example 4: When a body is earth-connected, electrons from the earth flow into the body. This means the body is
1) Unchanged
2) Charged positively
3) Charged negatively
4) An insulator
Solution:
When a positively charged body is connected to the earth, electrons flow from the earth to the body and the body becomes neutral.

Example 5: A conductor has $14.4 \times 10^{-19}$ coulombs positive charge. The conductor has (Charge on electron $=1.6 \times 10^{-19}$ coulombs )
1) 9 electrons in excess
2) 27 electrons in short
3) 27 electrons in excess
4) 9 electrons in short
Solution:
Electric charge
The loss of electrons gives a Positive charge
A positive charge shows the deficiency of electrons.
Number of electrons $=\frac{14.4 \times 10^{-19}}{1.6 \times 10^{-19}}=9$
Hence, the answer is the option (4).
Related Topics
Frequently Asked Questions (FAQs)
Electric charge is a conserved feature of certain subatomic particles that determines their electromagnetic interaction. Electric charge is divided into two types: positive and negative, which are carried by protons and electrons, respectively. When electrons are transported to or withdrawn from an object, an electrical charge is generated. When electrons are introduced to an object, it becomes negatively charged since electrons have a negative charge. When an object's electrons are withdrawn, it will become oppositely charged.
Formula for charge, Q=It
Properties of Electric charge:
A scalar quantity is charge. Charge is transferable; it can move from one body to another. Charge is constantly linked to mass, and similar electric charges repel one another. Electric charges that are opposite one other tend to attract each other.
Charge is a physical attribute that causes matter in an electromagnetic field to experience a force. In the presence of other matter with charge, charge is the fundamental feature of matter that exhibits electrostatic attraction or repulsion. When a charge is at rest, it only creates an electric field. Both an electric and a magnetic field are created when a charge moves.
The presence of an electric charge causes a modification in space known as the electric field. The electric force between a source charge and a test charge is mediated by the electric field. The field is a vector, thus it points away from positive charges and toward negative charges by definition.
The coulomb (C), named after French physicist Charles-Augustin de Coulomb, is the SI-derived unit of electric charge.