Antibody - Role of Antibodies, Structure, Types And Functions
Antibodies are special proteins produced by the immune system to fight against harmful substances called antigens, such as bacteria, viruses, and toxins. They are a key part of our body's defense and play an important role in identifying and neutralizing pathogens. Antibodies are produced by B-lymphocytes and are specific to the antigens they target. This concept is a vital part of Immunology, which helps us understand how our body fights diseases.
This Story also Contains
- What are Antibodies?
- Functions of Antibodies
- Antibody Structure
- Types of Antibodies and Their Functions
- Difference Between Antigen and Antibody
- Applications of Antibodies
- MCQs on Antibody
- Frequently Asked Questions (FAQs)
- Recommended Video on Antibody

Antibodies work with both innate immunity and adaptive immunity, where they circulate in body fluids to attack invaders. They are also involved in vaccination, where exposure to a harmless form of an antigen trains the body to produce antibodies for future protection. Learning about antibodies also helps in understanding AIDS and HIV, where the virus weakens the immune system and reduces the body’s ability to produce an effective antibody response.
What are Antibodies?
Antibodies are specialized proteins produced by the immune system to identify and neutralize foreign substances like bacteria and viruses. They bind specifically to antigens, marking them for destruction by other immune cells. They are also called immunoglobulins (Ig), are Y-shaped proteins made by the body's immune system or B cells. It can refer to the protein whether it's attached to a B cell (as a receptor) or free in the blood. It helps fight off diseases so that people do not get sick again with antigens they have been affected before. It functions by finding out what is harmful without harming healthy cells. They find out where things should not be then go ahead to kill them making sure the body is safe at all times.
Functions of Antibodies
The immune system uses antibodies for two main purposes , they identify antigens, and they alert other immune cells. Since an antibody attaches itself to a pathogen such as a virus, thereby identifying it as prey for macrophages or T-cells. This action helps other parts of the immune system destroy dangerous foreign organisms.
In fighting harmful invaders, the immune system needs to target them accurately and kill them effectively, which remains impossible without the help of such interactions. The five functions of antibodies in the immune system are:
Neutralization: Antibodies bind to pathogens (like viruses and bacteria) or toxins, blocking their ability to enter and infect cells.
Opsonization: By coating pathogens, antibodies make them easier for immune cells like macrophages to recognize and engulf, promoting phagocytosis.
Activation of the Complement System: Antibodies trigger the complement cascade, a series of proteins that help destroy pathogens through cell lysis and inflammation.
Agglutination: Antibodies bind to multiple pathogens, clumping them together to prevent their spread and make them easier to clear by immune cells.
Antibody-Dependent Cellular Cytotoxicity (ADCC): Antibodies mark infected cells, allowing natural killer (NK) cells to recognize and destroy these cells directly.
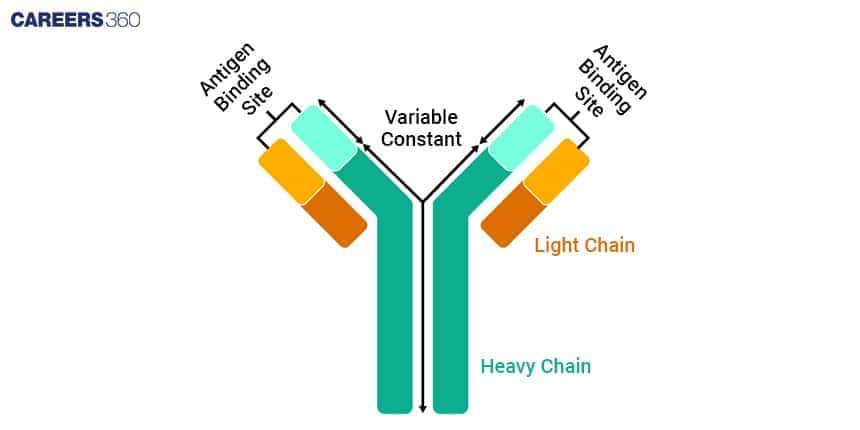
Antibody Structure
To understand how antibodies function in the immune system it’s important to know the structure of antibody. Their polypeptide chains and particular regions having been joined together create a distinctive Y-shape, which they use for finding and rendering harmless harmful substances called antigens.
Heavy and Light Chains
The structure of antibodies consist of four polypeptide chains, two heavy chains and two light chains. These chains are held together by disulphide bonds so that they form a Y-shaped structure.
Heavy Chains
The heavy chains are bigger polypeptides, which make up the antibody structure’s main section.
Every single heavy chain includes a variable area located at its peak as well as an unchanging part, occupying all other parts of this chain.
This IgG, IgA, IgM, IgE, or IgD determines the class of an antibody, such as the effector actions entailed in the latter while being present throughout its lifespan, which is determined by this consistent area.
Light Chains
Light chains are small polypeptides that are attached to heavyweight chains. Thus, two types exist, kappa (κ) and lambda (λ) which play similar roles in the binding of antigens. Each of these also comprises a variable portion and a constant fragment.
Variable and Constant Regions
Antibodies contain regions for variable (V) and constant (C) sections which respectively perform different roles within the operation of antibodies.
Variable Regions
Located at the ends of the Y-shaped structure are the variable regions, which are constituted by heavy and light chains.
It is these regions that distinguish them from one another in terms of specificity and allow them to attach to particular antigens.
The differences in amino acid sequences are what make variability possible in these areas leading to the production of an individualized distinctive antigen-binding site in them.
Significance in Antigen Binding
The point in the body where the antibody joins to the antigen is made up of the different regions on the heavy and light chains on the variable parts. That part of the antibody interacts physically with the antigen as it recognizes and binds to specific molecular structures. The wide range of antibodies produced by the immune system can specifically bind to many different antigens thanks to such a high degree of diversity.
Constant Regions
The rest of the antibody structure is composed of constant regions. In the heavy chains, the class of the antibody (IgG, IgA, IgM, IgE, or IgD) is determined by the constant region and the region is what enables effector functions e.g. binding to cell surface receptors and complement activation.
Significance in Immune Function
Determining the biological activity of the antibody, the constant regions are influential. For example, IgG antibodies protect a fetus by passing through a placenta, while IgA antibodies protect mucosal surfaces. Interactions between other immune system components such as phagocytes, and natural killer cells enhancing immune reactions involving the whole body also take place owing to this same region.
Types of Antibodies and Their Functions
There are five main different types of antibodies, namely IgG, IgA, IgM, IgE and IgD into which antibodies, sometimes referred to as immunoglobulins. Each of these types has its attributes and is located in different parts of the body whereby they perform different tasks during an immune response. Here is the description of antibody types and functions:
Antibody Type | Concentration in Blood | Main Functions | Primary Locations |
IgG | 75-80% | Long-term immunity, neutralizing toxins, opsonization | Blood, extracellular fluid, crosses the placenta |
IgA | 10-15% | Mucosal immunity, preventing pathogen attachment | Mucous membranes, saliva, tears, breast milk, respiratory tract, gastrointestinal tract |
IgM | 5-10% | Initial immune response, forming antigen-antibody complexes | Blood, lymphatic fluid |
IgE | <1% | Mediating allergic reactions, defending against parasites | Lungs, skin, mucous membranes |
IgD | <1% | Initiating and regulating immune responses | Blood, surface of B cells |
IgG antibodies
In the blood and extracellular fluids, IgG is the most common kind of antibody, accounting for roughly 75-80% of all antibodies in the human body.
The most important function of IgG is in protecting an individual against repeated infections due to pathogens, it provides long-term immunity and immune memory.
Its importance lies in its ability to neutralize toxins, tag pathogens for destruction by phagocytes (this process is called opsonization) and activate complement by all three pathways.
IgG antibodies, which are mainly found in the blood and extracellular fluid can cross the placenta to give the fetus passive immunity.
IgA antibodies
Immunoglobulin type A is about 10-15 per cent of all antibodies present in the body.
Its main role is safeguarding the body surfaces exposed to outside elements, i.e. mucosal immunity where its primary function is preventing pathogens from attaching themselves onto epithelial tissue found lining various body cavities such as the respiratory tract or intestines.
These are present in secretions like mucus, such as sweat or tears and also on the mucosa lining the gut and airways.
IgM Antibodies
IgM is one of the first categories of antibodies produced by the body when infections are detected.
This first type constitutes about 5-10 per cent of all antibodies present in the organism.
It acts mostly at its primary levels of defence against pathogen-causing agents by quickly forming immune complexes and initiating complement system activation through different pathways.
IgM can be predominantly located within blood vessels or in lymphatic vessels/fluids.
IgE antibodies
IgE exists at low levels within the circulating blood.
It is associated with allergic responses and fights off parasites.
It combines with antigens to stimulate the secretion of histamine from mast cells or basophils that cause allergy symptoms.
It is present in the lungs, skin and mucosa.
IgD Type Antibodies
IgD is the least known antibody and it comprises a small proportion of those present in the body.
It is primarily found on the surface of those who have not been exposed to immunogens.
Thus, its function is largely associated with triggering and controlling the immune system.
Besides being present in small quantities within the bloodstream, IgDs are also attached to those B-cell outer membranes.
Production of Antibodies
The process of the production of antibodies is given below:
B Cell Activation
The onset of activation starts with the interaction of various antigens (foreign substances) which then trigger a response from white blood cells called B-lymphocytes.
Antigen Recognition: B cells have B cell receptors that bind to specific antigens on their surface. When a receptor attaches antigen, it activates the B cell.
Helper T Cell Interaction: Activated B cells usually need more stimulation from helper T cells, which identify the antigen presented by B cells and secrete cytokines to promote B cell proliferation as well as differentiation.
Differentiation into Plasma Cells: After B cells have been activated they differentiate into plasma cells, whose main function is the large-scale production of antibodies that target the same antigen which has prompted them to be activated.
Clonal Selection and Expansion
Clonal selection is a vital process that ensures that the immune response discriminates highly against the invading pathogen.
Selection: Only B cells whose BCRs specifically join with the encountered antigen are selected for activation.
Clonal Expansion: Selected B cells proliferate and generate clones that extend with many identical B cells releasing the same specific antibody.
Memory B Cells: Among these clones, specific B cells turn into memory B cells residing for many years within the body and thus confer faster and stronger secondary responses to similar antigens.
Mechanism of Action of Antibodies
Antibodies work to make sure pathogens are removed and destroyed using different methods.
Neutralisation
When antibodies attach to pathogens, they counteract them and keep them from contacting cells in the same organism. This inhibits viruses and bacteria from going into and attacking cells, ensuring they do not cause harm.
Opsonisation
Pathogens are covered in antibodies during opsonisation. So phagocytes like macrophage cells or neutrophils can then destroy them. The Fc region of an antibody binds to Fc receptors that are located on phagocytes and therefore boosts the uptake and destruction of pathogens.
Complement Activation
Once antibodies attach to pathogens (such as bacteria), the first step is to trigger the activation process of a group of proteins termed complement to get lysed by them. This process kicks in when the proteins bond to antigens in what is referred to as the classical pathway for complement activation.
Agglutination
When antibodies join with antigens on the pathogens they stick the different pathogens together hence leading to agglutination i.e. clamping of pathogens. This makes it simpler for the phagocytes to decipher pathogens which makes them be removed easily from the body. The process by which antibodies join with pathogens in the process called agglutination which is known as clamping.
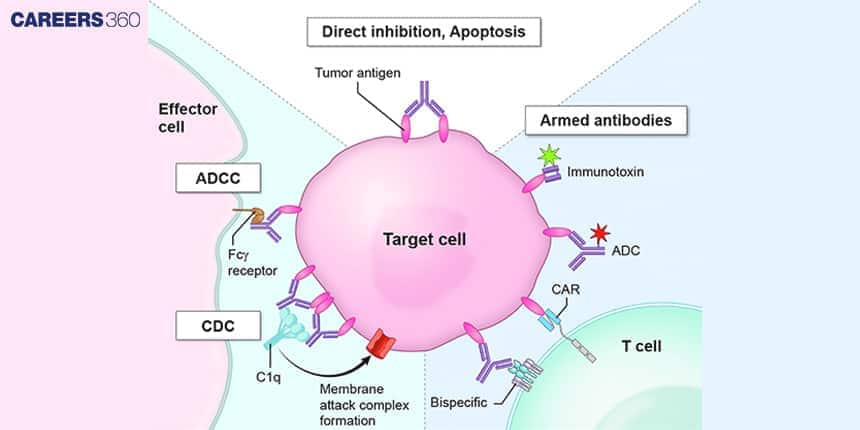
Antibody-Dependent Cellular Cytotoxicity (ADCC)
Antibodies in ADCC draw natural killer cells to destroy infected and malignant cells, with the antibody’s Fab region binding the antigen on the target cell and the Fc region binding it to Fc receptors on NK cells.
Epitope and Paratope
Epitope: The part of an antigen that is recognized and bound by an antibody or immune cell receptor. It's a small, specific region on the pathogen.
Paratope: The part of an antibody or immune receptor that binds to the epitope. It is specifically shaped to match the epitope, enabling a precise fit.
Difference Between Antigen and Antibody
Antigens and antibodies, addressing different roles in immunity and immune system. Knowing their differences is important if one hopes to understand how the body protects itself against harmful microbes.
Feature | Antigen | Antibody |
Definition | Substances that induce an immune response | Proteins produced in response to antigens |
Structure | Proteins, polysaccharides, or lipids | Y-shaped proteins with variable and constant regions |
Function | Initiate immune response | Bind specifically to antigens, neutralise, and mark for destruction |
Location | Found on pathogens or foreign substances | Produced by B cells, circulate in blood and body fluids |
Interaction | Recognised by immune cells | Bind to antigens to facilitate immune response |
Applications of Antibodies
There are numerous applications where antibodies are useful in diagnostics as well as treatment methods thereby serving as very useful instruments in today’s health sector. Their effectiveness arises from the fact that they attach themselves only to certain types of infections thus ensuring accurate scanning for particular illnesses before conveying necessary medications to infected areas.
Diagnostic Use of Antibodies
In various diagnostic tests, antibodies are important for detecting the existence of particular antigens that are linked to diseases.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is a frequently used test in scientific laboratories where it detects antigens through antibodies with relative ease. Its applications such as identifying viral proteins or antibodies against them from a patient’s circulating serum make it possible to diagnose diseases like hepatitis B virus infection, and AIDS and HIV infection among others.
Rapid Antigen Tests
Rapid antigen tests are utilized for identifying pathogens in point-of-care surroundings rapidly. They are crucial for the following reasons, especially in COVID-19 Testing: detecting SARS-CoV-2 antigens by using nasal or throat swabbing as well as checking if you have flu infection through the performance of rapid tests targeting viral protein in nasal or throat swabs.
Therapeutic Use of Antibodies
The treatment of some diseases has been improved greatly by monoclonal antibodies (mAbs) that allow for directing the therapy with a lot of precision.
Cancer Treatment
Monoclonal antibodies specifically target cancer cells and do not damage normal cells such as Rituximab (Rituxan) targets CD20 on B cells: It is the key element used to cure non-Hodgkin's lymphoma as well as chronic lymphocytic leukaemia.
Autoimmune Disorders
Monoclonal antibodies help to control the immune system in cases of autoimmune diseases. Used for the treatment of Crohn's disease or multiple sclerosis, Natalizumab, which is Tysabri, stops the migration of leukocytes to the brain.
Infectious Diseases
Additionally, monoclonal antibodies can be used to treat infectious illnesses. Palivizumab (Synagis) is used to treat the infection of respiratory syncytial virus in high-risk infants, for instance.
The diagram shows the monoclonal antibodies that are used in targeting cancer cells. They bind to a specific receptor located at the surface of such cells.
Antibody Engineering and Innovations
The development of new treatments has been completely changed by antibody engineering technology. The making of monoclonal antibodies and boosting of antibody properties are some of the achievements in this field.
Monoclonal Antibodies
Monoclonal antibodies are derived from a single clone of B cells. These antibodies are designed in such a way that they can attach themselves specifically to one type of antigen, hence offering targeted therapy for different diseases.
The treatment landscape has been revolutionised for many illnesses with the intervention of monoclonal antibodies since they are highly efficient and specific.
Genetic Engineering
Advances in genetic engineering have made antibodies more specific, with stronger bonds between antibodies and antigens. This resulted in the development of improved therapeutic modalities in the form of next-generation antibodies.
In phage display, bacteriophages (viruses that infect bacteria) are applied to target antigens to change the antibodies with high specificity. Phages are hung with the genes of the antibodies, which are then selected.
Bispecific antibodies are custom-made to attach two dissimilar antigens concurrently, so they can lock on two different targets or get two different cells to touch each other, e.g., T cells can contact tumour cells.
Resistance and Autoimmunity
Antibodies provides resistance to various pathogens and disease:
Pathogen Resistance
Antibody-based treatments’ efficacy could be compromised when pathogens become resistant to them, as is common with antibiotics-resistant pathogens due to mutations that occur.
Mechanisms of Resistance: Pathogens can escape antibody detection by altering their surface antigens or developing ways to break down and hide from antibodies.
Combatting Resistance: Methods of overcoming resistance include using combinations of antibodies that target various antigens, making antibodies that get conserved areas less susceptible to mutation, and applying next-generation sequencing to quickly detect and respond to new strains of resistant organisms.
Autoimmune Reactions
At times, antibody therapies can elicit autoimmune reactions, in which the immune system of an organism mistakenly assaults its cells or tissues.
Mechanisms: Autoimmunity could happen because a few cure antibodies will be able to cause reactions when they come into contact with human tissue or else mess up the body's natural defences.
Mitigation Strategies: In order to lower the chances of autoimmunity, there are three things that scientists do. They are making the specificity of antibodies better, carrying out preclinical trials carefully, and watching patients for adverse reactions. Another way to decrease immunogenicity is through humanising monoclonal antibodies.
MCQs on Antibody
Q1. Antibody is a ___ molecule.
Lipid
Protein
Nucleic acid
Carbohydrate
Correct answer: 2) Protein
Explanation:
Antibody (Ab) is also known as an immunoglobulin(Ig). These are large, Y-shaped blood proteins produced by plasma cells. They bind to foreign particles and invade them. These particles are foreign bodies that get attacked by Antibodies.
Antigens are foreign pathogens that invade the body and have the capability to give rise to a response from our immune system either by grouping up with a larger molecule or alone after binding with antibodies for a particular immune response. Hence, antigens stimulate the production of antibodies by the immune system.
Hence, the correct answer is option 2) Antibody is a protein molecule.
Q2. The antigen-binding site of an antibody is present at
The constant region
The C-terminal
The variable region
Between constant and variable region
Correct answer: 3) The variable region
Explanation:
The portion of the antibody that is subject to change and is in charge of identifying and attaching particular antigens—such as germs—is known as the variable region. All antibodies have the same constant area, which aids in antibody recognition by the body's immune cells. The antibody's C-terminal is merely its last segment and is not capable of binding to antigens. Although it aids in structure, the area between the constant and variable sections does not bind to antigens directly.
Hence, the correct answer is option 3) The variable region
Q3. The light chain and the heavy chain of the antibodies are joined by
Hydrogen bond
Ionic bond
Disulfide bond
Phosphodiester bond
Correct answer: 3) Disulfide bond
Explanation:
Antibodies, or immunoglobulins, are composed of two heavy chains and two light chains, which are held together by disulfide bonds. The disulfide bonds form between cysteine residues in the chains, providing structural stability to the antibody. The light chains are smaller in size and consist of one variable and one constant region, while the heavy chains are larger and have more constant regions. The variable regions of both chains together form the antigen-binding site, responsible for recognizing and binding to specific antigens. This structure allows antibodies to perform their immune functions, such as neutralizing pathogens or marking them for destruction by other immune cells.
Hence, the correct answer is option 3) Disulfide bond.
Also Read:
Frequently Asked Questions (FAQs)
Q1. What are antibodies?
Antibodies are proteins produced by B-lymphocytes that help recognize and neutralize harmful substances called antigens.
Q2. What is the structure of an antibody?
An antibody has a Y-shaped structure made of two light chains and two heavy chains, with specific antigen-binding sites.
Q3. What is the function of the antibody?
The main function of antibodies is to identify, bind to, and help remove antigens like bacteria and viruses from the body.
Q4. What are antibodies and their types?
Antibodies are immune proteins. The five main types of antibodies are IgA, IgG, IgM, IgE, and IgD.
Q5. Which antibody crosses the placenta?
IgG is the only antibody that can cross the placenta and provide immunity to the developing fetus.
Recommended Video on Antibody
Frequently Asked Questions (FAQs)
An antibody, also known as an immunoglobulin, is a protein released by the immune system with the aim of identifying and neutralizing foreign substances including bacteria and viruses. In order to neutralize antigens, it selectively binds to them.
Emil von Behring and Kitasato Shibasaburō found the antibodies at the very end of the 1890s. As they had discovered some substances, to be located within blood, neutralizing toxins, it opened a way for understanding immunity responses and more generally, antibodies.
IgA is found to be the first line of defense due to the reason that it protects the body from the entry and infection of mucosal surfaces by different foreign particles.
Monoclonal antibodies are a group of antibodies that are identical and are produced by a single clone of B cells. These cells act by simply binding to one definite antigen on its epitope. This feature has made them very useful in modern medicine, where they are used to provide highly specific treatments for diseases like cancer, autoimmune disorders and various infections.
Alteration of surface antigens by pathogens leads to therapy resistance as a result of versions in their surface protein structure or the development of means to dodge these antibodies. Measures to be taken here entail multiple antibody combinations targeting conserved areas less susceptible to mutation as well as fast recognition and response to additional resistant strains in any system through next-generation sequencing.
Antibodies, or immunoglobulins(Ig) are classified based on their H chains. IgG, IgM, IgA, IgD and IgE. There are five types of immunoglobulins.
Antibody is made up of variable regions and a constant region. The variable region is called so because it changes to various structures totally dependent upon the differences in the antigen. The constant region is called so because it cannot change its shape according to the antigen. These two chains are responsible for making the structure of antibodies.
IgE is the major antibody that plays a role in many allergic reactions where it binds to reintroduced antigens and focuses on the release of pharmacologically active agents. It also helps to show response to allergens and antigen preparation that is helpful in desensitization immunotherapy.
Functions of IgG
IgG antibodies give advantage of long-term protection against many different agents such as bacteria, viruses, and bacterial toxins.
IgG is found to be one of the most potent complement activators when taken in respect to all other antibodies.
The binding ability of IgG to antigens is more specifically effective because of the reason that it enhances phagocytosis.