Biomolecules- Carbohydrates, Proteins, Nucleic acids and Lipids
Biomolecules are chemical compounds present in living organisms, essential for structure and function. The sum total of different types of biomolecules, compounds and ions present in a cell is called a cellular pool. Biomolecules are compounds of carbon. Carbon is the most versatile and the most predominant element of life.
This Story also Contains
- What are Biomolecules?
- Classification of Biomolecules
- Carbohydrates
- Lipids
- Proteins
- Nucleic acids
- ATP – Energy Currency of the Cell
- Biomolecules NEET MCQs
Biomolecules are classified into micromolecules (water, minerals, amino acids, nucleotides) and macromolecules (carbohydrates, lipids, proteins, nucleic acids). The functional group determines their chemical properties. They first grow by chemical evolution. These biomolecules are fundamental building blocks of living organisms as they support the biological processes essential for life. Understanding biomolecules is key for NEET as they regulate metabolism, heredity, and energy flow.
What are Biomolecules?
A biomolecule is a chemical compound found in living organisms or a molecule produced by a living organism. Biomolecules have a wide range of sizes and structures. Biomolecules are mainly classified into two types: micromolecules and macromolecules. Micromolecules are small-sized whereas macromolecules are large-sized. A biomolecule is generally identified based on its molecular form, and a chemical equation usually represents a molecular form.
Classification of Biomolecules
Based on the size and molecular weight, the biomolecules are divided into two parts:
Micromolecules
Micromolecules contain small-sized, low molecular weights between 18 and 800 daltons. They are found in the acid soluble pool. Minerals, water, sugars, amino acids, and nucleotides come under micromolecules.
Micromolecules | Features |
Minerals |
|
| |
| |
Nucleotide |
|
Macromolecules
Macromolecules are large-sized and have a high molecular weight. These are above 10000 daltons. They are found in acid insoluble pool. Carbohydrates, lipids, proteins, and nucleic acids come under macromolecules. Macromolecules are formed by polymerizing subunits called monomers, except lipids. Macromolecules are alternatively called polyanions.
Carbohydrates
Carbohydrates are made up of simple sugars such as glucose, fructose, and galactose. Complex carbohydrates like starch and glycogen are made up of long chains of glucose molecules. Carbohydrates are involved in the structural components of cells and organisms, such as the cell wall of plants and the exoskeletons of insects.
Cane sugar (sucrose), glucose , starch, etc. Some of the carbohydrates, which are sweet in taste, are also called sugars. The general formula for carbohydrates is Cx(H2O)y, usually considered as carbon hydrates.
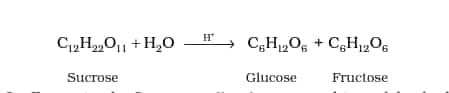
Based on their behaviour during hydrolysis, carbohydrates are classified into monosaccharides, oligosaccharides, and polysaccharides.
A carbohydrate which cannot be hydrolysed further to give a simpler unit of polyhydroxy aldehyde is called a monosaccharide.
Carbohydrate that yields two to ten monosaccharide units, on hydrolysis are called oligosaccharide
Polysaccharides are carbohydrates which yield a large number of monosaccharide units on hydrolysis.
Based on the number of carbon atoms and the functional group present in them Monosaccharides are further classified into different categories. For example, if a monosaccharide contains an aldehyde group, it is known as an aldose, and if it contains a keto group, it is known as a ketose.
Lipids
Lipids are another important class of biomolecules that play a crucial role in the structure and function of cell membranes. They are insoluble in water and include fats, oils, waxes, and steroids. Fats and oils are made up of fatty acids and glycerol, while waxes are made up of long-chain fatty acids and alcohol.
Lipids with a higher melting point are usually solids at room temperature and are called fats or waxes; at a lower melting point, they are usually liquids at room temperature and are called oils. Lipids store energy in the body, insulate organs and tissues and act as signalling molecules.
Proteins
Proteins are the most important biomolecules in living organisms. Milk, cheese, pulses, peanuts, fish, meat, etc. are a few examples of foods that contain protein. Proteins are polypeptide chains, which are generally made up of amino acids. Amino acids are further divided into two kinds.
Essential amino acids
Non-essential amino acids
Glycine, alanine, and serine are examples of amino acids. Proteins are mainly used to fight against infectious organisms and transport nutrients across the membrane. Based on the structure of the protein, it is classified into four types.
Primary structure: It is the basic structure of the protein
Secondary structure: This structure protein threads form a helix
Tertiary structure: It is folded upon itself like a hollow woollen ball
Quaternary structure: each polypeptide develops its tertiary structure and functions as a protein
Basis of their molecular shape Proteins can be classified into two types
Fibrous proteins: When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, a fibre-like structure is formed which is called Fibrous protein.
Globular proteins: When the chains of polypeptides coil around to give a spherical shape Globular proteins are formed.
Nucleic acids
Nucleic acids are large biomolecules. A significant function of nucleic acids is the storage and expression of genomic information. Nucleic acids are one of the most important macromolecules for life. They will carry the genetic blueprint of a cell and have instructions for the functioning of the cell. The two examples of nucleic acids are deoxyribonucleic acid and ribonucleic acid.
Deoxyribonucleic acid (DNA): C10H12N2O4, also written as (C5H5N5O)n where n is the number of nucleotide units in the DNA strand
Ribonucleic acid (RNA): C10H12N2O5P, also written as (C5H4O)n(C10H12N2O)nP where n is the number of nucleotide units in the RNA strand.
ATP – Energy Currency of the Cell
ATP is a biomolecule that functions as the primary energy source for cellular processes in living organisms. It consists of a nucleotide base (adenine), a sugar molecule (ribose), and three phosphate groups. The high-energy phosphate bonds between the phosphate groups store energy that can be used by cells to power various processes, such as muscle contraction, nerve impulse transmission, and chemical synthesis. ATP is synthesized during cellular respiration and is used immediately, so it is constantly recycled in the cell. The chemical formula for ATP is C10H16N5O13P3.
Biomolecules NEET MCQs
Q1. The cellular pool comprises hundreds of organic and inorganic biomolecules. The organic molecules include
Carbohydrates
Proteins
Nucleic acids
More than one correct
Correct answer: 4) More than one correct
Explanation:
The organic molecules present within the cell are categorized into:
1. Carbohydrates:
- Monosaccharides (e.g., glucose) are simple sugars serving as energy sources.
- Polysaccharides (e.g., starch, glycogen) are complex carbohydrates providing both energy and structural support to cells.
2. Proteins:
- Composed of amino acids, proteins are fundamental to various cellular activities such as enzyme function, structural integrity, signal transmission, and material transport.
3. Lipids:
- This group encompasses fats, phospholipids, and steroids which are crucial for energy storage, maintaining cell membrane structure, and participating in signaling pathways.
4. Nucleic Acids:
- DNA and RNA are the genetic material, essential for storing, transferring, and synthesizing proteins according to genetic instructions.
Hence, the correct answer is option 4) More than one option is correct.
Q2. Lecithin, a small molecular weight organic compound found in living tissues, is an example of:
Amino Acids
Phospholipids
Glycerides
Carbohydrates
Correct answer: 2) Phospholipids
Explanation:
Some lipids have phosphorous and a phosphorylated organic compound in them. These are phospholipids. They are found in cell membranes. Lecithin is one example.
Option (3) is incorrect, as glycerides are another group of lipids in which both glycerol and fatty acids are present.
Option (1) and (4) are incorrect as amino acids and carbohydrates are separate groups of biomolecules.
Hence, the correct answer is option 2) Phospholipids
Q3. Anabolic reactions are _____ in nature and cell anabolism cellular size ____.
Exergonic, decreases
Spontaneous, decreases
Endergonic, remains the same
Endergonic, increases
Correct answer: 4) Endergonic, increases
Explanation:
Protein synthesis and DNA production are examples of anabolic reactions, which create larger molecules from smaller ones. Because energy is necessary for these reactions to take place, they are endergonic, or energy-absorbing.
Because new molecules are being produced and added to the cell as a result of these processes, the cell gets bigger. Growth and repair depend on this.
Hence, the correct answer is option 4)Endergonic, increase.
Also Read:
Frequently Asked Questions (FAQs)
Amino acid micromolecules are joined together by peptide bonds to form polypeptide chains.
Adenosine Triphosphate.
Deoxyribonucleic acid is known as DNA, and ribonucleic acid is known as RNA.
Living organisms obtain essential amino acids along with food.
The first amino acid of the sequence is called the N-terminal amino acid, and the last amino acid is called the C-terminal amino acid of a peptide chain.
Glycogen is called animal starch, the reserve food material for animals, bacteria, and fungi.