Enzyme: Definition, Mechanisms, Nomenclature, Structure, Classification, and Function
Essential proteins that increase the rate of chemical reactions in living organisms, contributing to metabolic activities as well as digestion and respiration are termed enzymes. Enzymes is a topic of the chapter Cell: The Unit of Life in Biology.
This Story also Contains
- What are Enzymes?
- Structure and Function
- Enzyme Structure
- Enzymes classification
- Mechanism of Enzyme Action
- Enzyme Kinetics
- Enzyme Inhibition
- Applications of Enzymes
- Recommended video for Enzymes
What are Enzymes?
Enzymes are very selective, all are aimed at performing a specific biochemical reaction within cells and are very particular in their actions. They are very vital for keeping the body balanced and facilitating other processes in the bodies of living organisms.
The history of enzymes begins at the end of the nineteenth century and among the first scientists who researched this phenomenon, Eduard Buchner who established that yeast extracts can carry out sugar fermentation, separately from living yeast cells, which proved the emergence of enzymatic activity. Since then enzymes have been used in various formulations of biochemical reactions leading to improvements in medicine, industries and biotechnological fields.
Structure and Function
The structure and function of enzymes are listed below
Chemical Nature of Enzymes
Proteins and RNA molecules as enzymes:
Enzymes are mostly composed of proteins; however, some ribonucleic acids (RNA) called ribozymes can catalyze reactions as well. There are proteins obtained from amino acids which are polymerized in a certain manner that defines the size and shape, and the ability to act as a catalyst.
Composition and structure:
All enzymes usually have quaternary structures which are very important in the catalytic roles of the enzymes. The place on the enzyme where the substrate is bound and where the chemical transformation takes place is frequently a small indentation or a groove in the protein structure of the enzyme. This structure enables enzymes to selectively bind onto the substrates to accomplish the catalysis of the chemical reactions crucial in the life processes.
Also Read-
Enzyme Structure
The structure of the enzyme is listed below
Primary, secondary, tertiary, and quaternary structures
Enzyme structure is defined at multiple levels:
Primary structure is the sequence of the amino acids followed by the secondary structure which is the folding of the protein into alpha helix and beta sheets while tertiary structure is the Australia of the protein due to bonds like hydrogen and disulphide bridges while quaternary structure has to do with the formation of multiple polypeptides that forms complex enzymes.
Active site and substrate specificity
The active site of an enzyme is the place on the enzyme where substrate molecules are attached and a chemical reaction takes place. Enzymes are stereospecific which implies that they only attach to a certain substrate in compliance with the shapes and chemical properties of the substrate.
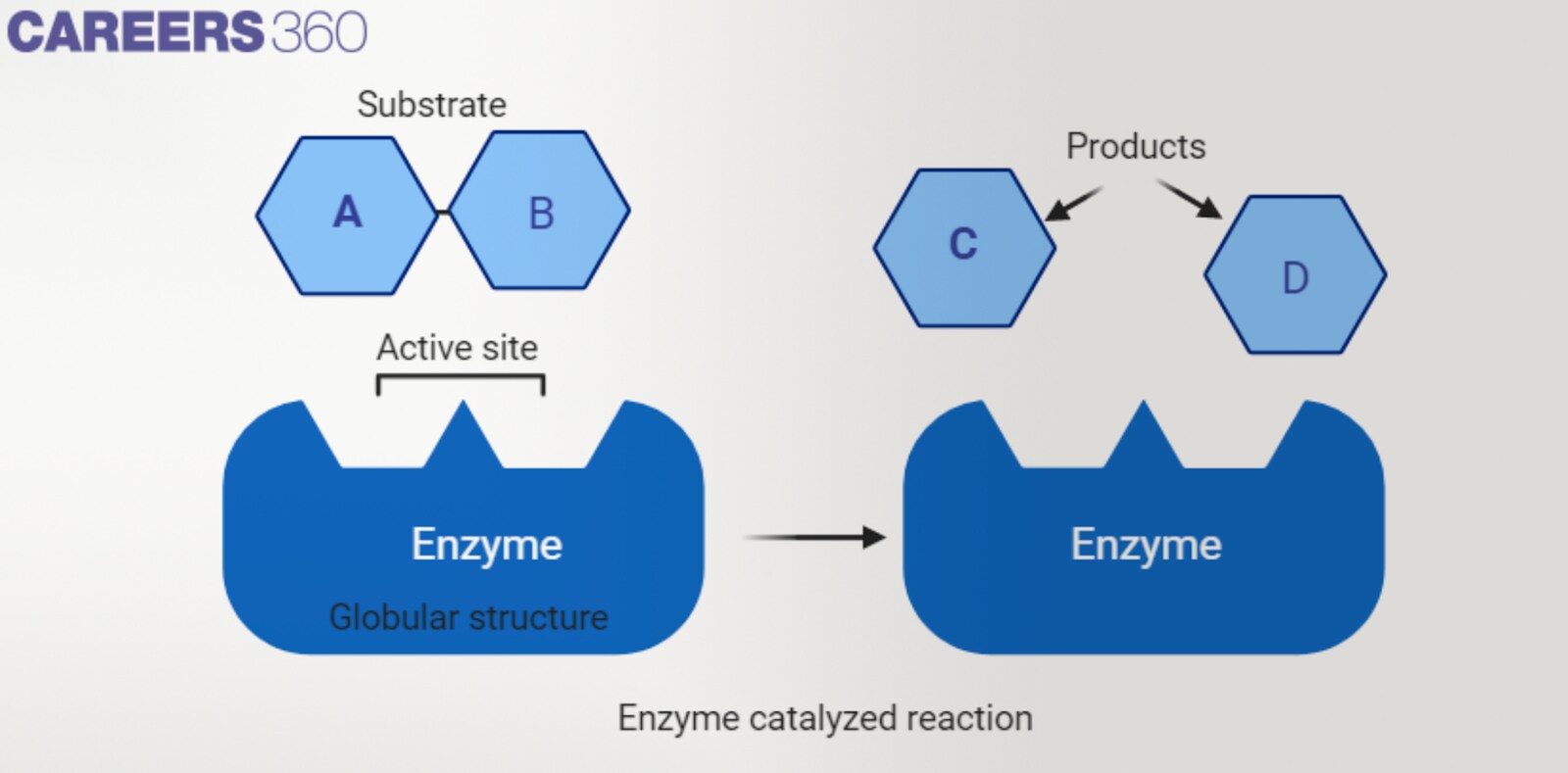
Enzymes classification
The classification of enzymes is discussed below
Classification of Enzyme based on function
Oxidoreductases
Oxidoreductases are involved in oxidation/reduction reactions where one molecule is oxidized by having its electrons removed while the other molecule is reduced by gaining the electrons.
These include enzymes for powerhouse processes like the electron transport chain during cellular respiration.
Examples include dehydrogenases that pass hydrogen atoms (and their electrons) to the substrates.
Transferases
Transferases include enzymes that either add an amino, acyl or phosphate group to a molecule or remove it to another molecule.
They are needed in metabolic processes, particularly in the biosynthesis of amino acids, nucleotides and lipids.
An example is kinases, enzymes that donate phosphate groups from ATP to substrates in signal transduction or cellular regulation.
Hydrolases
Hydrolases involve chemical reactions where a substrate undergoes hydrolysis which is the breaking of chemical bonds by the use of water molecules.
Examples include participation in the digestion, and degradation of food molecules, participation in the recycling of proteins and signal transduction which involves the removal of phosphate groups from the proteins.
It may be proteases that break down proteins; lipases that break down lipids or phosphatases that remove phosphate groups.
Lyases
Lyases break the chemical bonds in molecules and make new double bonds, or add groups to double bonds without the processes of hydrolyzing or oxidation.
They carry out processes that are in the metabolic maps such as the citric acid cycle which involves cleavage of citrate into oxaloacetate and acetyl-CoA.
For instance, there are decarboxylases which remove carboxyl groups and dehydratases which remove water molecules.
Isomerases
Enzymes that change the position of atoms in the molecule transforming one isomer into another are called isomerases.
That is, they are essential for supporting the metabolism and homeostasis of cells by converting substances into their active forms.
Examples include glucose-6-phosphate isomerase that catalyses the interconversion of glucose-6-phosphate with fructose-6-phosphate during glycolysis.
Ligases
Ligases catalyse the formation of a molecule from two smaller molecules, usually using up ATP or other nucleotide triphosphate.
They are involved in DNA synthesis, and repair, as well as in the biosynthesis of many complex molecules in which new covalent bond has to be created.
Illustrations include DNA ligases which it is involved in suturing together two DNA strands during DNA replication or DNA repair mechanism.
Mechanism of Enzyme Action
The mechanism of enzyme action involves several models that describe how enzymes interact with substrates to catalyze chemical reactions:
Lock and Key model
By the lock and key structure, it is argued that an enzyme has a rigid active site that is complementary to the substrate molecule in form, shape and size, so a key fits into a lock.
This model indicates that the enzyme and substrate have to groove complementary to each other from the onset, so are selective and fast in catalyzing reactions.
When a substrate molecule is attached to the active site the enzyme helps to transform the substrate into the product by stabilizing the transition state and reducing the activation energy.
Induced Fit model
In the induced fit model, it is believed that the active site of the enzyme is flexible and changes shape upon the binding of the substrate.
Also, at the beginning of the interaction, the enzyme and substrate may not fit each other perfectly.
As the substrate is bound to the enzyme, it undergoes a conformational change to gain a better match of the enzyme and the substrate.
This induced fit mechanism helps to improve the solid and appropriate catalytic site, brings stability to the transition state, and brings about the change of the substrate into the product.
Enzyme substrate complex formation
In catalysis, enzyme-substrate complex formation is the bonding between the enzyme and substrate molecules for a limited amount of time.
When the substrate molecule comes across the enzyme it fits into the active site of the enzyme, and the two join to form the enzyme-substrate complex.
Based on the structures and properties mentioned, this complex maintains different noncovalent bonding sources; for example, hydrogen bonds, electrostatic interactions, and van der Waals interactions.
At the active site of the enzyme-substrate complex, the enzyme enables the change of the substrate into the product since the energy that is needed to complete this process is reduced.
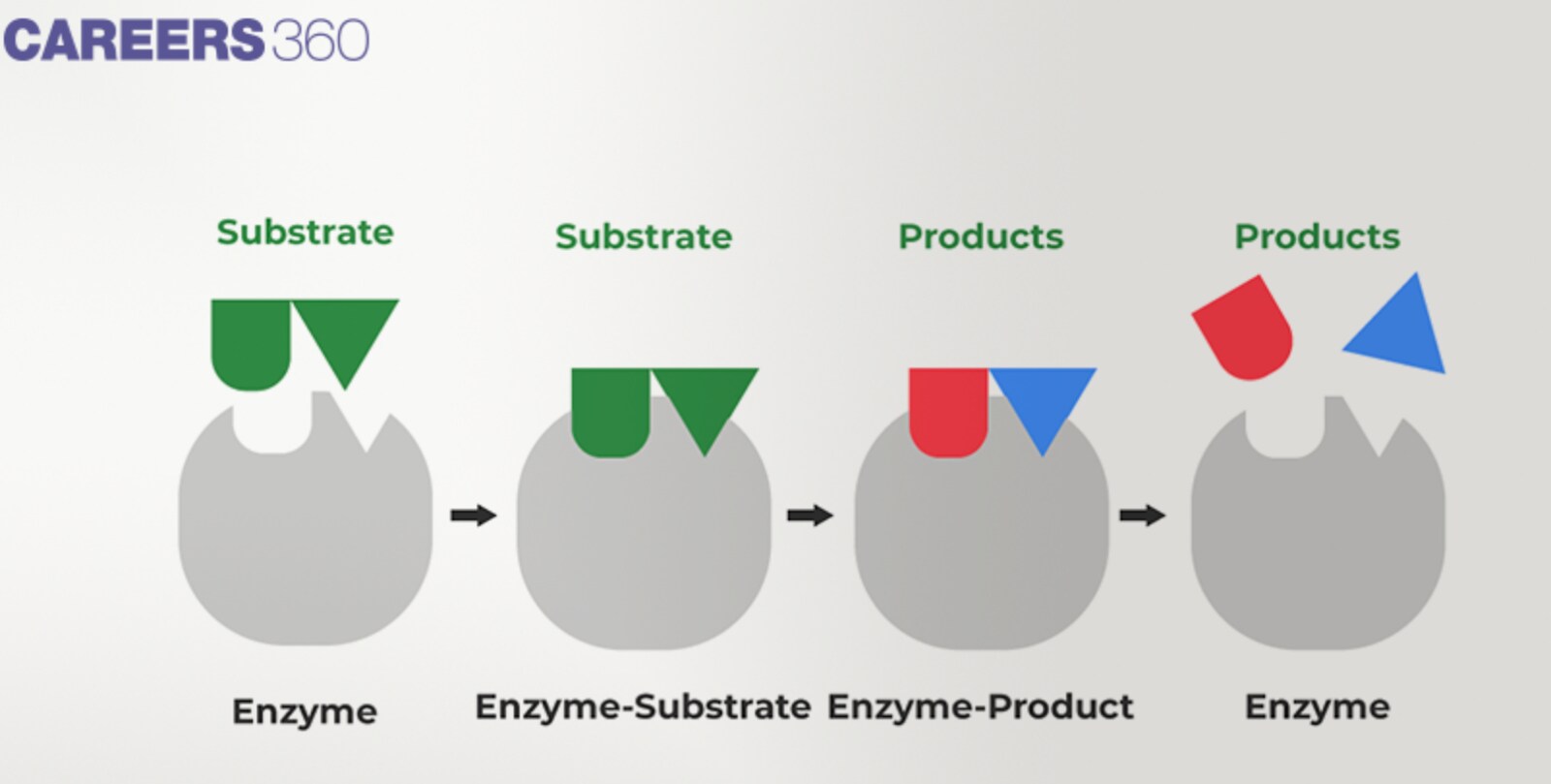
Enzyme Kinetics
The enzyme kinetics is discussed below
Factors Affecting Enzyme Activity
Temperature
The temperature factor has an optimal range within which the enzyme is most active, and this is usually 37°C for human enzymes.
Very high or very low temperatures can inactivate enzymes permanently we call this process denaturation.
pH
Different enzymes have specific pH values due to the constitution of amino acids within the active site of the enzymes.
pH variations usually cause instability to a protein by affecting hydrogen bonding and electrostatic forces which are essential in enzyme functioning.
Substrate concentration
- Enzyme activity rises directly with the substrate concentrations up to the point of demasking the maximum rate (Vmax) where all the enzyme’s active sites are occupied by the substrate molecules.
- As discussed earlier, beyond Vmax, there is no effect of increasing the relative concentration of the substrate in the reaction rate.
Enzyme concentration
- This is because higher enzyme concentrations imply a greater number of active sites for the attachment of the substrate thus enhancing the rate of reaction.
- Activity may level off if the substrate concentration reaches its low amount even when the enzyme is plentiful in the solution.
Inhibitors and activators
- Inhibition can be of the reversible competitive or noncompetitive type in which inhibitors directly interact with enzymes thereby decreasing their activity.
- To respond to this, activators are known to attach to enzymes and confirm their classical code, their functionality, high efficiency, and speeds of reactions.
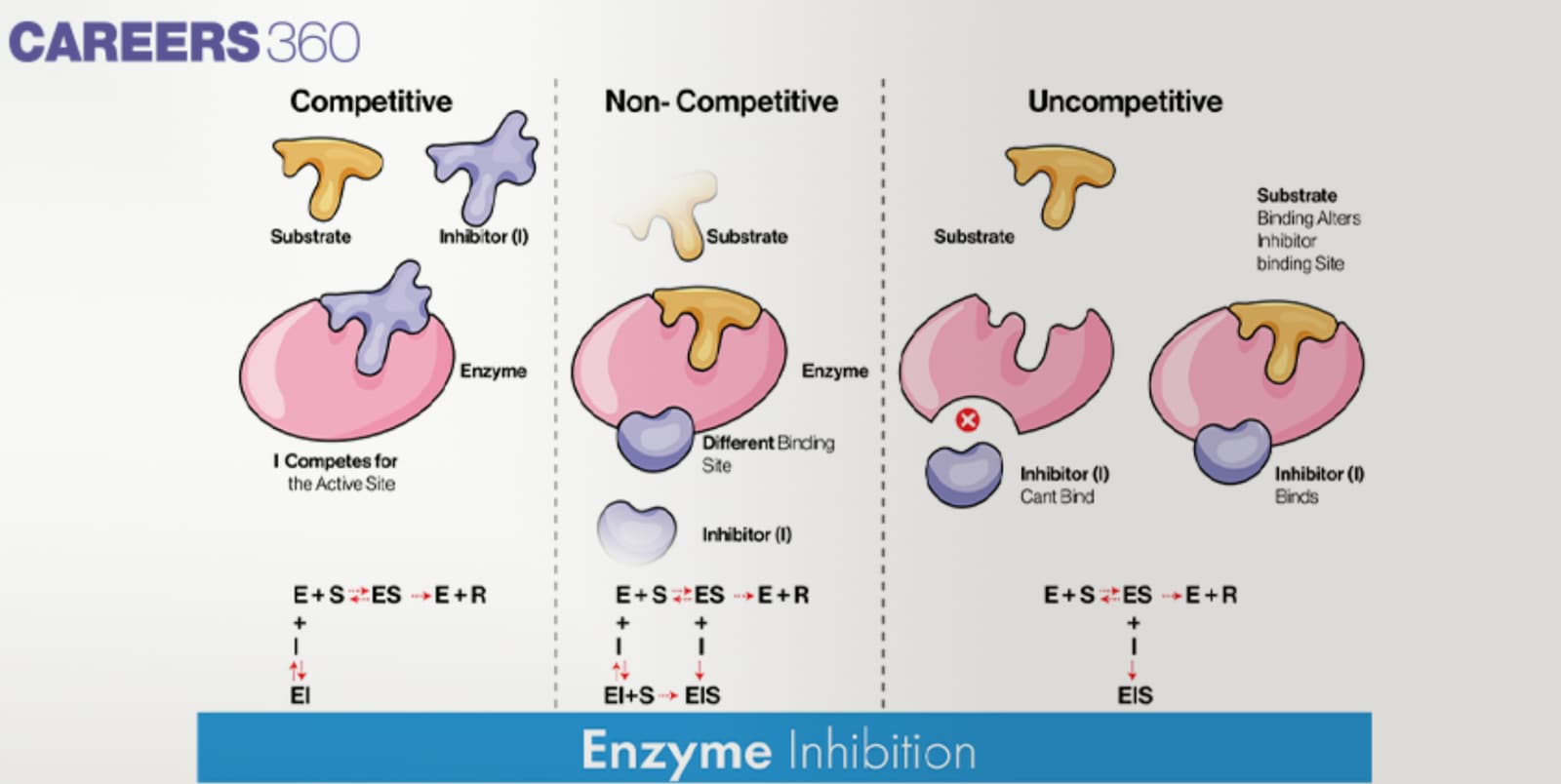
Enzyme Inhibition
The enzyme inhibition is discussed below
Types of Inhibition
Competitive inhibition
Competitive inhibitors can accommodate themselves into the active site of the enzyme preventing substrate approach.
These substances bind themselves to the enzyme and directly fight the substrate for the chance to bind onto the active site.
High concentrations of substrate can dispel competitive inhibition in that they reduce the opportunity of the inhibitor to compete for the active site.
Noncompetitive inhibition
Noncompetitive inhibitors bind to distinct sites on the enzyme not at the active site of the enzyme.
In this case, it can be seen that this binding alters the conformation of the enzyme and hence a reduction in the activity of the enzyme is observed.
Noncompetitive inhibition can be Irreversible and does not involve direct competition with the substrate; it cannot be removed by raising the concentration of the substrate because it does not affect the Ki value.
Uncompetitive inhibition
Some are uncompetitive inhibitors because they only attach themselves to the enzyme-substrate complex rather than the free enzyme.
This binding normally takes place at the allosteric site and it causes a change in the enzyme’s active site’s binding capacity to the substrate.
Uncompetitive inhibitors decrease the Vmax as well as the Km of the enzyme and thus decrease the velocity of the reaction.
Allosteric inhibition
These bind at other sites of the enzyme and the altering of the shape of the enzyme in the process reduces effectiveness.
In this binding, the activity of the enzyme in question can either be reduced or enhanced depending on the nature of the compound referred to as the allosteric modulator.
Allosteric regulation is reversible and depends on the signal in the cell or something that the cell requires.
Applications of Enzymes
The applications of enzymes are discussed below:
Industrial Applications
In food processing, enzymes are used to improve such aspects of food as taste, texture or even nutrient content in food products. Detergents especially enzymes; enhance the cleaning requirement and limit the usage of raw materials that are so damaging to articles. The existing uses of enzymes in textiles are for wearing and creasing fabrics or for making stonewashed denim and in paper industries, they help in bleaching pulp and modifying the fibres.
Medical Applications
Diagnostic test equipment involves enzymes since it is easy to diagnose diseases through tests involving enzymes. Lysosomal storage disorders are some of the diseases treated by enzyme replacement therapies since the bodies of affected individuals lack specific enzymes. Supplements for the enzymes also aid people suffering from pancreatic or other digestive diseases to be able to digest food and assimilate nutrients seemingly suitable for their body type.
Environmental Applications
A few examples of bioremediation include enzymes which are used in the degradation of substances such as oil and industrial chemicals in the environment. They also find application in wastewater treatment because they enhance the rate of decomposition of organic matter, hence the impact on the environment is minimized. In biofuel production, enzymes transform biomass into biofuels including ethanol hence providing a better option in comparison to the fossil products.
Also Read-
Recommended video for Enzymes
Frequently Asked Questions (FAQs)
Competitive inhibition takes place when a molecule binds to the active site of an enzyme thus competing with the substrate. Non – Noncompetitive inhibition is where another particle will bind to another site on the enzyme altering its conformation and reducing its activity without hindering the active site.
Enzymes find use in disease and pathogen identification and are vital in modern diagnostics. They are also applied in enzyme replacement therapy in genetic disorders and medicines for drug identification and synthesis.
Future studies concerning enzymes will be related to the improvement of the activity and selectivity of enzymes through engineering and design. Thus, the future offers more opportunities for inventions in sustainable resources, technologies, and biomedical and biotechnological areas like the field of personalized medicine due to increasing knowledge of the operation of enzymes and potentials in enzyme regulation.
Enzymes in the environment are used in bioremediation, projects on removing pollutants and improving the efficiency of waste treatment. In conclusion, enzymes represent an inspiration for efficiency and a prime example of sustainability in nature; they are positively contributing to the development of technology, health care, and the protection of the environment. Their uses remain diverse and keep on being developed to further improve different fields in the global community.
Temperature and pH affect the activity of the enzyme in terms of the velocity of the inherent chemical reaction and the best velocity is reached when the enzyme is in its optimal condition. Between such values, enzymes can precipitate and thus lose their conformation and hence their ability to function. Fluctuations in temperature and pH levels impact the stability of the enzyme as well as the capability of the enzyme to bind to its substrates.