Nitrogen Metabolism: Definition, Process, Examples, Types, & Facts
What Is Nitrogen Metabolism?
Nitrogen metabolism incorporates all the processes that convert nitrogen to assorted chemical forms, enabling its assimilation into living organisms. Nitrogen is important for living organisms because it is a part of the amino acids, nucleic acids, and other cell constituents.
The nitrogen cycle encompasses all the processes involved in the conversion of nitrogen in the environment, which includes nitrogen fixation carried out by the bacteria, assimilation by the plants, and incorporation into the tissue of the animals while returning to the atmosphere through the excretion of the animals and denitrification. This cycle subsequently renews all the reservoirs from which many life forms can access, hence indicating that it is highly fundamental to the life-sustaining process of the ecosystems.
Commonly Asked Questions
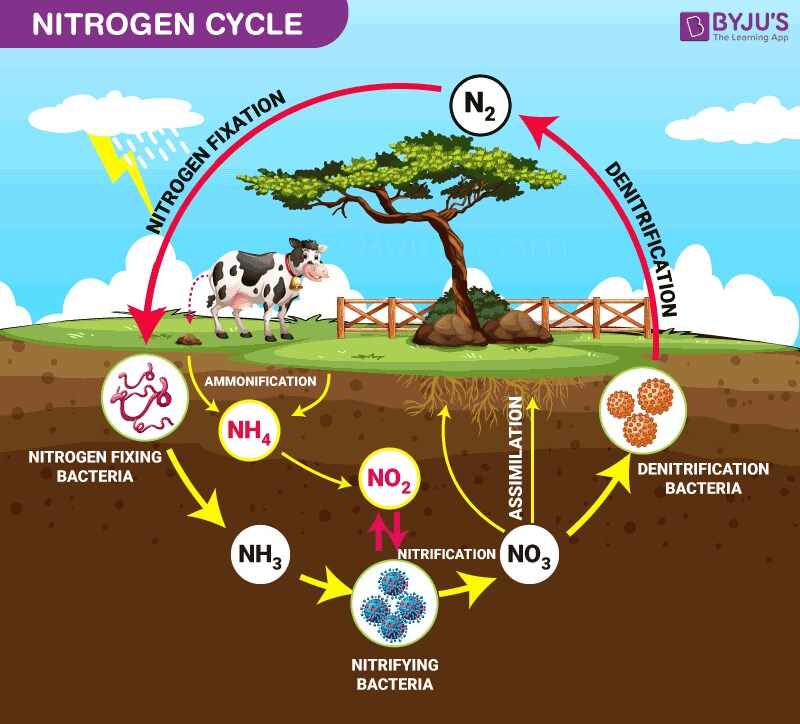
Nitrogen Cycle
The major components of the Nitrogen Cycle are:
Nitrogen fixation
Conversion of gaseous nitrogen (N₂) of the atmosphere to ammonia (NH₃) by nitrogen-fixing bacteria and archaea, largely symbiotic with the roots of plants, including legumes.
Nitrification
Biological oxidation of ammonia to nitrite (NO₂⁻) catalyzed by nitrifying bacteria, followed by oxidation of nitrite to nitrate (NO₃⁻).
Assimilation
Uptake of ammonia, nitrite, or nitrate by plants and other organisms to synthesize organic nitrogen compounds, e.g., amino acids.
Ammonification
The organic nitrogen is changed back to ammonia or ammonium ions (NH4+) by the decomposer microorganisms. Thus, it changes nitrogen back to the soil.
Denitrification
Nitrate is reduced back to gaseous nitrogen (N2) or nitrous oxide (N2O) by the denitrifying bacteria by releasing it into the atmosphere.
Diagram Of The Nitrogen Cycle
Commonly Asked Questions
Nitrogen Fixation
Nitrogen fixation is classified into:
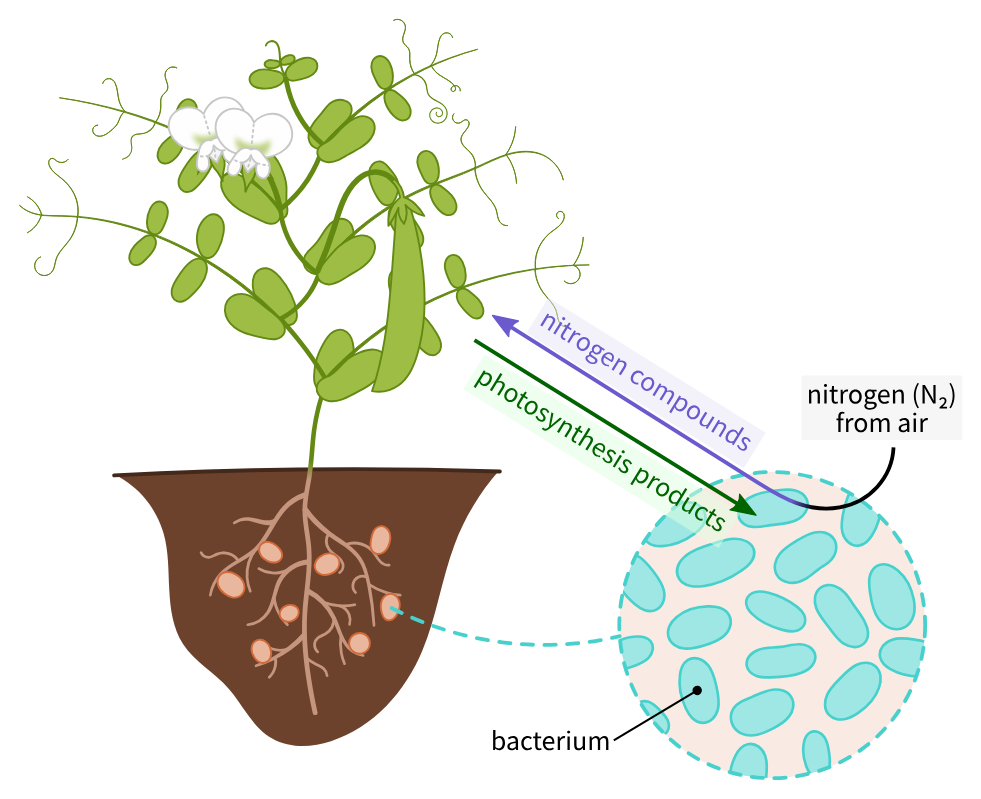
Biological Nitrogen Fixation
The bacteria Rhizobia enter into symbiosis with legumes, which inhabit the root nodules where they convert atmospheric nitrogen (N₂) to ammonia (NH₃).
Process of nitrogenase enzyme
Nitrogen-fixing bacteria have the nitrogenase enzyme complex, which catalyzes the reduction process of N₂ to NH₃.
Being very energy-consuming, it normally requires chemical energy provided by ATP.
Diagram Of Nitrogen Fixation In Root Nodules
Abiotic Nitrogen Fixation
Lightning
The fuel of lightning splits the triple bond of N₂, so nitrogen can be combined with oxygen to form nitrogen oxides NOx, a substance that can be dissolved in rain to form nitrate.
Industrial fixation (Haber-Bosch process)
The Haber-Bosch process combines atmospheric nitrogen and hydrogen gas at high pressure and temperature with the help of an iron catalyst, producing ammonia. It is an essential step in the formation of fertilizer.
Commonly Asked Questions
Nitrogen Metabolism In Animals
The nitrogen metabolism in animals includes:
Protein Metabolism
Proteins are broken down into amino acids via proteolytic enzymes present in the stomach and small intestine.
Thereafter these amino acids are transferred to the bloodstream through intestinal walls.
Amino acids undergo deamination in the liver in that the amino group is removed. This leads to the formation of ammonia (NH₃) and a keto acid.
Ammonia is very poisonous and cannot be directly excreted.
Urea Cycle
The urea cycle, also known as the ornithine cycle, is an ammonia detoxification process taking place in the liver to change ammonia into urea.
The urea, being a less toxic compound, can then be carried in the blood safely out of the body to the kidneys to be removed.
The liver synthesized urea carried in blood to the kidneys to be excreted from the body in urine
Commonly Asked Questions
Frequently Asked Questions (FAQs)