Calcium Carbonate - Structure, Formula, Preparation, Properties, Uses, FAQs
Calcium carbonate is an inorganic chemical compound. The chemical formula of calcium carbonate CaCO3 or CaCO3 where calcium formula is Ca. Calcium carbonate is a chemical compound that is one of the most commonly seen chemical compounds. It can also be found in the earth's crust. It can also be found in a variety of different materials, such as marbles and limestone. Despite the fact that they come in numerous forms, they are chemically similar and only differ physically. Calcium carbonate, which is non-toxic, odorless which occurs naturally in the form of limestones, chalks, marbles, as well as pearls. The limestone formula or limestone chemical formula is the same as CaCO3.
This Story also Contains
- Structure of calcium carbonate (CaCO3)
- Occurrence of calcium carbonate
- Preparation of CaCO3
- Commercial production of calcium carbonate
- Properties of calcium carbonate (CaCO3)
- Application of calcium carbonate

The active element in agricultural lime is calcium carbonate, which is formed when calcium ions combine with carbonate ions in hard water to generate limescale. It can be used as a calcium supplement or an antacid in the medical setting, although excessive ingestion can be harmful and lead to poor digestion. Since the chemical name of chalk is calcium carbonate thus calcium carbonate common name is chalk.
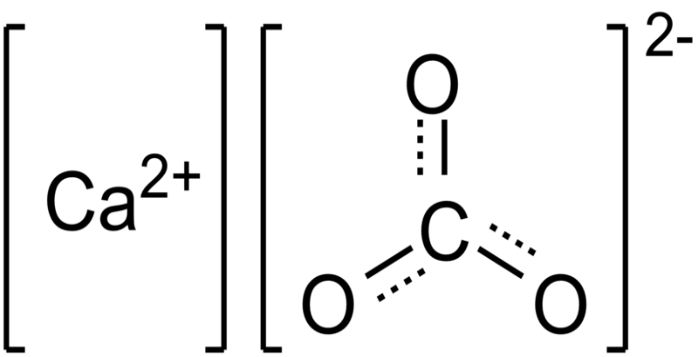
Structure of calcium carbonate (CaCO3)
Under normal conditions, CaCO3 (the mineral calcite) has a thermodynamically stable hexagonal shape. Other forms, such as denser 2.83g/cm3 orthorhombic CaCO3 as well as hexagonal CaCO3, are possible. It is feasible to prepare aragonite at temperatures exceeding 85°C along with vaterite at 60°C, for example. Calcite has calcium atoms coordinated with six oxygen atoms, while aragonite has nine oxygen atoms coordinated. The vaterite's chemical composition is unknown.
Magnesium carbonate (MgCO3) has a calcite structure, but strontium carbonate (SrCO3) and barium carbonate (BaCO3) have an aragonite structure, which reflects their greater ionic radii.
Occurrence of calcium carbonate
- Geological Sources: Calcite, aragonite, and vaterite are pure calcium carbonate minerals found in nature. Limestones, chalks, travertines, and marbles are examples of industrially significant calcium carbonate source rocks. Corals thrive in warm, clear tropical waters rather than the frigid waters of the polar regions. Since the ample light and filterable food, calcium carbonate sources such as planktons (coccoliths and planktic foraminifera), sponges, echinoderms, coral algae and mollusks are typically found in shallow water locations.
- Biological Sources: Calcium carbonate is found in eggshells, snail shells, and most seashells, and it is also a commercial source of this chemical. Oyster shells have recently been recognized as a dietary calcium source as well as a useful industrial source. On a diet, dark green vegetables like broccoli and kale contain a lot of calcium carbonate, but they aren't useful as an industrial supply.
Also read -
Commonly Asked Questions
Preparation of CaCO3
- Carbon dioxide and slaked lime are used as raw materials to make CaCO3. Calcite is formed when carbon dioxide is transported through slaked lime.
Adding sodium carbonate to calcium chloride is another way to produce calcite.
Ca (OH)2 + CO2 → CaCO3 + H2O
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
- Calcium hydrogen-carbonate is formed when there is an excess of carbon dioxide in the atmosphere. It's made by passing carbon dioxide gas through calcium hydroxide on a big scale (slaked lime). Carbon dioxide, on the other hand, creates the soluble calcium hydrogen-carbonate when it is released in excess.
Ca(OH)2 + CO2→ CaCO3 + H2O
Commercial production of calcium carbonate
Commercially available calcium carbonate comes in two grades. In the industrial world, both classes compete primarily on particle size and product attributes.
- Ground Calcium carbonate: This is made by extracting and processing naturally occurring calcium carbonate deposits. The form of GCC crystals is irregularly rhombohedral, with a wider size distribution.
CaCO3+H2SO4 → CaSO4+H2O+CO2
- Precipitated Calcium carbonate: It is produced by a by-product of some bulk chemical operations. The shape of PCC crystals varies depending on product, along with particle is more homogenous as well as regular, with restricted size distribution.
PCC has smaller particles, is purer, is less abrasive, and has a brighter appearance than GCC.
CaCO3 Limestone CaO+CO2
Calcium carbonate produces carbon dioxide when it reacts with dilute acids.
CaCO3 + 2HCl → CaCl2 + H2O +CO2
Related Topics |
Commonly Asked Questions
Properties of calcium carbonate (CaCO3)
- Calcium carbonate is a fluffy calcium powder that decomposes into carbon dioxide when heated to 1200 K.
- When it reacts with dilute acid, it produces carbon dioxide as a by-product.
- At a temperature of 1200 K, calcium carbonate decomposes into calcium oxide and carbon dioxide.
- When carbon dioxide reacts with dilute acids, calcium carbonate is generated.
- At a temperature of 1200 K, calcium carbonate decomposes into calcium oxide and carbon dioxide.
- When carbon dioxide reacts with dilute acids, calcium carbonate is generated.
- Calcium carbonate (CaCO3) has a molecular weight of 100.0869 g/mol.
- CaCO3 has a molar mass of 100.0869 g/mol.
Application of calcium carbonate
- The pulp and paper industry uses a lot of calcium carbonate. It can be used as a filter and a pigment, resulting in a whiter, higher-quality pigment than other minerals.
- It is used in construction industry as filler in concrete to improve its durability and aesthetics, as well as to cleanse metals for use in construction applications.
- Calcium carbonate is also used in fertilizers to deliver calcium to plants and to keep the pH of the soil stable.
- Calcium carbonate can be used as a supplement in vitamins and as a food ingredient for livestock and people.
- Calcium carbonate is used in water and wastewater treatment plants to remove acidity and pollutants.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Commonly Asked Questions
Frequently Asked Questions (FAQs)