Carbocation - Definition, Types, Formation, Order and Stability
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. We can say that they are carbon cations. Formerly, it was known as carbonium ion. Carbocation today is defined as any even-electron cation that possesses a significant positive charge on a carbon atom. Talking about some general characteristics, the carbon cations are very reactive and unstable due to an incomplete octet. In simple words, carbocations do not have eight electrons; therefore, they do not satisfy the octet rule. In carbocation, the hybridization of carbon will be sp2 and its shape is trigonal planar.
This Story also Contains
- Carbocation-
- Solved Examples Based On Carbocation
- Conclusion
In this article, we will cover the topic (carbocation). This topic falls under the broader category of (Some Basic Principles of Organic Chemistry), which is a crucial chapter in (Class 11 Chemistry). It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Carbocation-
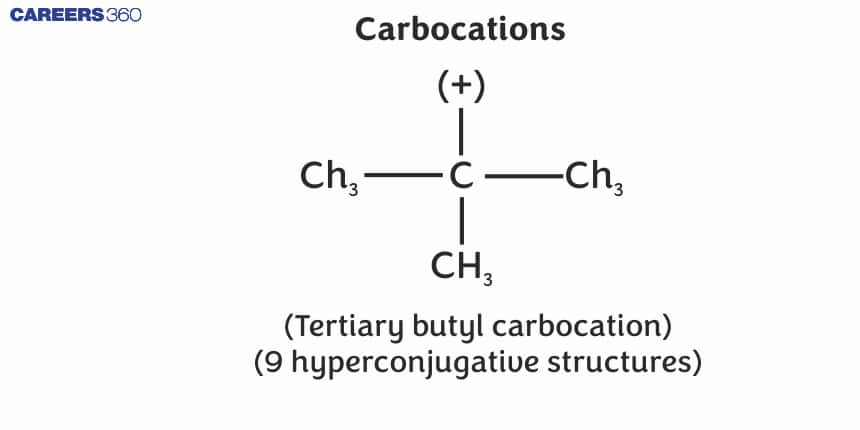
A species having a carbon atom possessing a sextet (6 in a group) of electrons and a positive charge is called a carbocation (earlier called carbonium ion). The ${ }^{+} \mathrm{CH}_3$ ion is known as a methyl cation or methyl carbonium ion. Carbocations are classified as primary, secondary or tertiary depending on whether one, two or three carbons are directly attached to the positively charged carbon. Carbocations are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects. The observed order of carbocation stability is:${ }^{+} \mathrm{CH} 3<\mathrm{CH}_3 \mathrm{C}^{+} \mathrm{H}_2<\left(\mathrm{CH}_3\right)_2 \mathrm{C}^{+} \mathrm{H}<\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+}$.These carbocations have a trigonal planar shape with positively charged carbon being sp2 hybridised. Thus, the shape of ${ }^{+} \mathrm{CH}_3$may be considered as being derived from the overlap of three equivalent $C\left(s p^2\right)$hybridised orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as $\mathrm{C}\left(s p^2\right)-\mathrm{H}(1 \mathrm{~s})$ sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons. The shape of methyl carbocation is given as below:

Recommended topic video on ( Carbocation)
Solved Examples Based On Carbocation
Q.1 The shape of carbocation is
(1) pyramidal
(2) bent
(3) linear
(4) trigonal planar
Solution:
As we learned -
Carbocation -
Those chemical species have trivalent carbon and bear a positive charge.
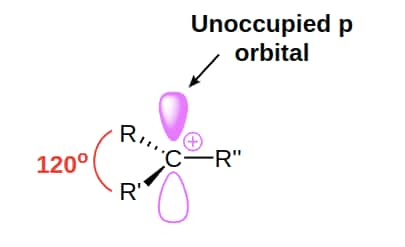
A carbocation is sp2 hybridised which has a trigonal pyramidal shape. In carbonation, a carbon atom bears three bonds and a positive charge.
Therefore, option (4) is correct.
Q.2 The order of stability of the following carbocations is :

(1) $III>I>II$
(2) $III>II>I$
(3) $II>III>I$
(4) $I>II>III$
Solution:
As we learned -
The order of stability of carbocation is -

So, III > I > II
Therefore, option (1) is correct.
Conclusion
Carbocations are carbon atoms in an organic molecule bearing a positive formal charge. Therefore they are carbon cations. Carbocations have only six electrons in their valence shell making them deficient. Thus, they are unstable electrophiles and will react very quickly with nucleophiles and will react to form new bonds. Because of their reactivity with heteroatoms, carbocations are very useful intermediates in many common organic reactions.