Nucleophiles and Electrophiles
The word electrophile is made from “electro”, derived from electron and “phile”, which means loving Electrophiles can accept a couple of electrons. Those reactants that are either positively charged or neutral, with no lone pair of electrons, are referred to as electrophiles. These chemical species, which are positively charged or electron-deficient, can accept electron pairs from other molecules or atoms. Any molecule, ion or atom that is deficient in electrons in some manner can act as an electrophile. In other words, the reagent which attacks the negative of the molecule or loves electrons is called electrophile.
This Story also Contains
- Nucleophiles and Electrophiles
- Some Solved Examples
- Conclusion
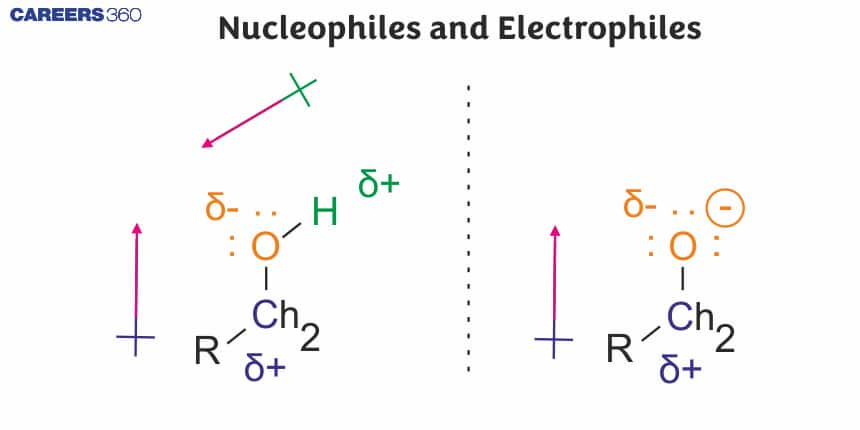
The word nucleophile is made from two words “Nucleo”, derived from the nucleus and “phile”, which means loving. Species that attack the positive side of the substrate or love the nucleus are called nucleophiles. A nucleophile is a reactant which gives an electron pair to form a covalent bond. A nucleophile is usually charged negatively or is neutral with a lone couple of donatable electrons.$\mathrm{H} 2 \mathrm{O},-\mathrm{OMe}$ or -OtBuare some examples. Overall, the electron-rich species is a nucleophile. They remain dormant in the molecule because they are not needed. As a result, this type of chemical species can be attracted to the positive area of another compound or molecule.
In this article, we will cover the topic (Nucleophiles and Electrophiles). This topic falls under the broader category of (Some Basic Principles of Organic Chemistry), which is a crucial chapter in (Class 11 Chemistry).
Nucleophiles and Electrophiles
A reagent that brings an electron pair to the reactive site is called a nucleophile(Nu: i.e, nucleus seeking and the reaction is then called nucleophilic.
A reagent that takes away an electron pair from the reactive site is called electrophile (E+) i.e., electron seeking and the reaction is called electrophilic.
During a polar organic reaction, a nucleophile attacks an electrophilic centre of the substrate which is that specific atom or part of the substrate which is electron deficient. Similarly, the electrophiles attack at the nucleophilic centre, which is the electron-rich centre of the substrate. Thus, the electrophiles receive electron pair from the substrate when the two undergo bonding interaction. A curved-arrow notation is used to show the movement of an electron pair from the nucleophile to the electrophile.
Some examples of nucleophiles are the negatively charged ions with lone pair of electrons such as hydroxide$=\left(\mathrm{HO}^{-}\right)$, cyanide $\left(\mathrm{NC}^{-}\right)$ions and carbanions $\left(\mathrm{R}_3 \mathrm{C}^{-}\right)$. Neutral molecules such as $\mathrm{H}_2 \mathrm{O}, \mathrm{R}_3 \mathrm{~N}, \mathrm{R}_2 \mathrm{NH}_2$etc., can also act as nucleophiles due to the presence of lone pair of electrons.
Examples of electrophiles include carbocations $\left({ }^{+} \mathrm{CH}_3\right)$ and neutral molecules having functional groups like carbonyl group $(>\mathrm{C}=\mathrm{O})$ or alkyl halides $\left(\mathrm{R}_3 \mathrm{C}-\mathrm{X}\right.$, where X is a halogen atom). The carbon atom in carbocations has sextet configuration; hence, it is electron-deficient and can receive a pair of electrons from the nucleophiles.
In neutral molecules such as alkyl halides, due to the polarity of the$\mathrm{C}-\mathrm{X}$ bond, a partial positive charge is generated on the carbon atom and hence the carbon atom becomes an electrophilic centre at which a nucleophile can attack
Recommended topic video on(Nucleophiles and Electrophiles)
Some Solved Examples
Q.1 Which species represents the electrophile in aromatic nitration?
$\begin{aligned} & \text { (1) } N O_2 \\ & \text { (2) } N O_3 \\ & \text { (3) } N O_3 \\ & \text { (4) } N O_2^+\end{aligned}$
Solution:
As we have learned
Positively charged electrophiles -
Chemical species having capacity to attack e- rich portion of substance.
- wherein
$\mathrm{H}^{+}, \mathrm{Cl}^{+}, \mathrm{Br}^{+}, \mathrm{NO}^{+}, \mathrm{NO}_2^{+}, \mathrm{R}^{+}$ $R-\stackrel{\oplus}{C}=O$
In aromatic nitration, the reagent $\mathrm{NaNO}_2 / \mathrm{HCl}$ gives $\mathrm{NO}_2^{+}$ electrophile (positively charged electrophile) which reacts with substrate to give nitro product
Therefore, option (4) is correct.
Q.2 The increasing order of nucleophilicity of the following nucleophiles is :
a) $\mathrm{CH}_3 \mathrm{CO}_2$
(b) $\mathrm{H}_2 \mathrm{O}$
c) $\mathrm{CH}_3 \mathrm{SO}_3$
d) OH
(1) $(a)<(d)<(c)<(b$
(2) $(b)<(c)<(d)<(a)$
(3) $(d)<(a)<(c)<(b)$
(4) $(b)<(c)<(a)<(d)$
Solution:
As we have learned
Nucleophile:-
Those electron-rich chemical species have the capacity to attack the electron-deficient portion of the substrate.
Nucleophile must have complete octet and at least one lone pair
(a)  (b) $\mathrm{H}_2 \mathrm{O}$ (c)
(b) $\mathrm{H}_2 \mathrm{O}$ (c) (d) $\mathrm{OH}^{-}$
(d) $\mathrm{OH}^{-}$
The order of Nucleophilicity is
$\mathrm{OH}^{-}>\mathrm{CH}_3 \mathrm{COO}^{-}>\mathrm{CH}_3 \mathrm{SO}_3^{-}>\mathrm{H}_2 \mathrm{O}$
Both (a) and (c) are resonance stabilized ions, so, donating tendency of electrons on oxygen is reduced, but delocalisation of electrons is more in $\mathrm{CH}_3 \mathrm{SO}_3$. Thus, it is a weak nucleophile than $\mathrm{CH}_3 \mathrm{COO}$. Further, it has been found that a reactive atom bearing a negative charge is always a better nucleophile than the same neutral atom.
Thus, OH- is a better nucleophile than$\mathrm{H}_2 \mathrm{O}$.
Therefore,$d>a>c>b$
Therefore, option (4) is correct.
Q.3 In a nucleophilic substitution reaction :
$\mathrm{R}-\mathrm{Br}+\mathrm{Cl}^{-} \xrightarrow{D M F} R-\mathrm{Cl}+\mathrm{Br}$
which one of the following undergoes complete inversion of configuration?
(1) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHC}_6 \mathrm{H}_5 \mathrm{Br}$
(2) $\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}$
(3)$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH} \mathrm{CH}_3 \mathrm{Br}$
$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH} \mathrm{CH}_3 \mathrm{Br}$
Solution:
As we have learned
It is a$S_N 2$ type of reaction that requires a less crowded substance. Among the given options$\mathrm{C}_6 \mathrm{H}_5-\mathrm{CH}_2-\mathrm{Br}$ is the least crowded substrate.
Nucleophilic substitution reactions that lead to inversion in configuration follow $S_N 2$a mechanism. Since it involves the formation of a transition state which is crowded by 5 groups attached to a carbon atom, steric hindrance of a major factor in these kinds of reactions. Due to steric hindrance of the benzene ring, options (1) and (4) are ruled out. Among (2) and (3), (3) has a bulkier group attached to the C having Br. It also has 3 hydrogens compared to 2. Thus (3) will offer more steric hindrance than (2). Thus (2) will give inversion in the configuration.
Hence, the answer is the option (2).
Conclusion
Electrophiles and nucleophiles are crucial chemical species involved in forming new chemical bonds by accepting or donating electrons, respectively.
Electrophiles:
- Derived from "electro" (electron) and "phile" (loving).
- Positively charged or neutral with no lone pairs of electrons.
- Electron-deficient and can accept electron pairs from other molecules or atoms.
- Attack negative parts of molecules or love electrons.
Nucleophiles:
- Derived from "nucleo" (nucleus) and "phile" (loving).
- Negatively charged or neutral with lone pairs of donatable electrons.
- Electron-rich species that give an electron pair to form a covalent bond.
- Examples include$\mathrm{H}_2 \mathrm{O}_2-\mathrm{OMe}$, and -OtBu.
- Attack positive parts of substrates or love the nucleus.
Hence, Electrophiles accept electrons due to their low electron concentration, while nucleophiles donate electrons to form chemical bonds.