1. Who discovered cathode rays?
Sir J.J Thomson discovered cathode rays.
2. What are cathode rays?
Cathode rays are the beam of negatively charged particles called as electrons which are travelling to the positively charged anode situated towards the end of the vacuum tube from the negatively charged cathode.
3. What did sir J.J Thomson discover?
Sir J.J Thomson discovered the negatively charged particles known as the electrons for which he won the Nobel prize.
4. What is the nature of the cathode rays? Are they positive or negative in nature?
The nature of charge present in the cathode rays are negative as they are made up of negatively charged particles called as electrons.
5. What is cathode ray oscilloscope?
A cathode ray oscilloscope is an appliance which has general applications in a laboratory to exhibit or to calculate and examine the various types of waves of electrical circuit. The cathode ray oscilloscope is a very rapid X-Y plotter that can show a signal which is input versus another signal or time.
6. How to discharge a cathode ray tube?
In order to discharge a cathode ray tube to release an electron it is first detached from the atoms present in the cathode region.
7. What are the characteristics of the cathode ray tube?
The cathode rays comprise of negatively charged particles known as electrons.
As they get strike by the particular substance they generate energy in the form of heat.
They cause ionization of gases as they pass through it.
X-rays are produced if they are collided by the target.
The material used in the discharge tube and nature of the gases present does not affect the nature of the cathode rays.
- A black coloured spot is produced on a photographic plate when they are incident on it.
8. What is the meaning of evacuated tube?
Before the application of the cathode ray tube in experiment it is first evacuated or drained out the air present inside completely to keep the pressure inside at low levels. Thus, the draining out of the air inside the tube is called an evacuated tube.
9. What is the modern-day name for the cathode rays?
The modern-day name for the cathode rays is called electrons which are negatively charged in nature and are found in the orbit of the atom.
10. What are cathode rays made up of?
The cathode rays are made up of negatively charged particles called as electrons.
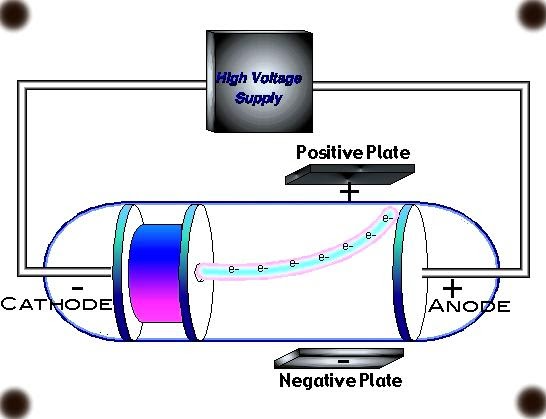
11. How did Thomson use electric and magnetic fields in his experiment?
Thomson applied both electric and magnetic fields perpendicular to the path of the cathode rays. By observing how these fields deflected the rays, he could determine that the rays were made of negatively charged particles. The degree of deflection helped him calculate the charge-to-mass ratio of these particles.
12. How did Thomson determine that cathode rays were negatively charged?
Thomson determined that cathode rays were negatively charged by observing their deflection in electric and magnetic fields. The rays were attracted to the positively charged plate and repelled by the negatively charged plate, indicating a negative charge. This behavior was consistent across different cathode materials and gas types.
13. What was the significance of the fluorescent screen in Thomson's experiment?
The fluorescent screen in Thomson's experiment allowed him to visually observe the path of the cathode rays. When the rays hit the screen, they produced a glowing spot, making it possible to track their deflection under various conditions and draw conclusions about their nature.
14. What was the significance of the charge-to-mass ratio in Thomson's experiment?
The charge-to-mass ratio was a crucial measurement in Thomson's experiment. It showed that the particles in cathode rays had a much higher charge-to-mass ratio than any known ion, suggesting they were a fundamental component of atoms. This led to the discovery of the electron and revolutionized our understanding of atomic structure.
15. How did Thomson's experiment disprove the idea that atoms were indivisible?
Thomson's experiment showed that cathode rays consisted of negatively charged particles (electrons) that could be separated from atoms. This discovery demonstrated that atoms were not indivisible, as previously believed, but contained smaller subatomic particles. It marked a significant shift in our understanding of atomic structure.
16. What was the role of the cathode in Thomson's experiment?
The cathode in Thomson's experiment served as the source of electrons. When a high voltage was applied, the cathode emitted electrons through a process called thermionic emission. The material of the cathode was varied in different experiments to show that electrons were a universal component of all matter, regardless of the source.
17. What was the significance of Thomson's use of both electric and magnetic fields in his experiment?
Thomson's use of both electric and magnetic fields was crucial for determining the nature of cathode rays. By comparing the deflections caused by each field, he could confirm that the rays consisted of particles rather than waves. Additionally, using both fields allowed him to calculate the charge-to-mass ratio of the particles, a key measurement in identifying them as electrons.
18. What role did gas discharge play in Thomson's cathode ray experiment?
Gas discharge was crucial in Thomson's experiment as it provided the source of electrons. When a high voltage was applied across the cathode ray tube containing a small amount of gas, it caused the gas to ionize. The positively charged gas ions then bombarded the cathode, causing it to emit electrons, which formed the cathode ray beam.
19. How did Thomson's cathode ray experiment relate to the concept of ionization?
Thomson's experiment provided insights into the process of ionization. The cathode rays he studied were essentially beams of electrons that had been separated from atoms, creating positive ions in the process. This helped explain how atoms could become charged and laid the groundwork for understanding phenomena like the ionization of gases and the behavior of ions in solution.
20. How did Thomson's experiment contribute to the understanding of plasma?
While Thomson's primary focus wasn't on plasma, his work with ionized gases in cathode ray tubes was an early step in understanding plasma physics. The behavior of electrons and ions in his experiments provided insights into the nature of ionized gases, which are the basis of plasma. This laid groundwork for later studies in plasma physics and its applications.
21. What role did the vacuum play in Thomson's cathode ray tube?
The vacuum in Thomson's cathode ray tube was essential for the experiment's success. It allowed the cathode rays (electrons) to travel freely without colliding with gas molecules, ensuring clear observations of their behavior under electric and magnetic fields. The vacuum also prevented the oxidation of the cathode.
22. What was the role of the anode in Thomson's cathode ray tube?
The anode in Thomson's cathode ray tube served as the positive electrode. It attracted the electrons emitted by the cathode, accelerating them and forming the cathode ray beam. The anode often had a small hole in its center, allowing a portion of the electron beam to pass through for observation and experimentation.
23. How did Thomson's cathode ray tube differ from earlier versions?
Thomson's cathode ray tube was more sophisticated than earlier versions. It included deflection plates and a fluorescent screen, allowing him to observe and measure the behavior of cathode rays under the influence of electric and magnetic fields. This design was crucial for determining the charge-to-mass ratio of the particles.
24. Why was it important that Thomson used different materials for the cathode in his experiments?
Thomson used different materials for the cathode to prove that electrons were universal constituents of all matter. By showing that the same negatively charged particles were produced regardless of the cathode material, he demonstrated that electrons were fundamental particles present in all atoms, not just a property of specific elements.
25. What were some of the challenges Thomson faced in conducting his experiment?
Thomson faced several challenges in his experiment, including maintaining a high vacuum in the cathode ray tube, precisely controlling and measuring the electric and magnetic fields, and accurately observing and measuring the deflection of the cathode rays. He also had to develop new mathematical techniques to analyze his results and draw meaningful conclusions.
26. What was the main purpose of J.J. Thomson's cathode ray experiment?
The main purpose of J.J. Thomson's cathode ray experiment was to investigate the nature of cathode rays and ultimately discover the electron. Thomson aimed to determine whether cathode rays were particles or waves, and to measure their properties such as mass and charge.
27. What was the "plum pudding" model of the atom, and how did it relate to Thomson's experiment?
The "plum pudding" model was Thomson's proposed atomic structure based on his cathode ray experiment. In this model, the atom was envisioned as a positively charged "pudding" with negatively charged electrons (the "plums") embedded within it. This model was a direct result of Thomson's discovery of the electron and his understanding of atomic neutrality.
28. How did Thomson's experiment relate to the work of William Crookes?
Thomson's experiment built upon the work of William Crookes, who had previously studied cathode rays. Crookes developed improved vacuum tubes that allowed for better observation of cathode rays, but he didn't determine their nature. Thomson used a modified version of Crookes' tube to identify the particles as electrons and measure their properties.
29. How did Thomson's experiment influence the development of atomic theory?
Thomson's experiment was a pivotal moment in the development of atomic theory. By discovering the electron, he showed that atoms were divisible and had internal structure. This led to a shift from Dalton's indivisible atom concept to more complex models of atomic structure, paving the way for later discoveries like the atomic nucleus and the development of quantum mechanics.
30. How did Thomson's experiment relate to the concept of quantization of charge?
Thomson's experiment provided early evidence for the quantization of charge. By determining the charge-to-mass ratio of electrons and later estimating the charge of an electron, Thomson's work suggested that electric charge came in discrete units. This concept was later refined by Robert Millikan's oil drop experiment, confirming the quantized nature of electric charge.
31. How did Thomson's experiment contribute to the understanding of isotopes?
While Thomson's original experiment didn't directly lead to the discovery of isotopes, his work on positive rays (a follow-up to his cathode ray experiments) did. Using similar principles, he observed that neon produced two different parabolas in his apparatus, suggesting atoms of the same element could have different masses. This observation was crucial in the later discovery and understanding of isotopes.
32. How did Thomson's experiment contribute to the understanding of atomic mass?
Thomson's experiment didn't directly measure atomic mass, but it provided crucial insights. By determining the charge-to-mass ratio of electrons, Thomson showed that electrons were much lighter than atoms. This suggested that the majority of an atom's mass must be contained in its positive portion, leading to further investigations into atomic structure and mass distribution.
33. How did Thomson's experiment contribute to the development of mass spectrometry?
Thomson's experiment laid the groundwork for mass spectrometry. His method of using electric and magnetic fields to deflect charged particles based on their mass-to-charge ratio became the fundamental principle of mass spectrometers. This technique is now widely used in various scientific fields for analyzing the composition of substances.
34. What was the significance of Thomson's discovery in terms of understanding electrical conductivity?
Thomson's discovery of the electron provided a fundamental explanation for electrical conductivity. It showed that electricity was the flow of these negatively charged particles (electrons) through materials. This understanding revolutionized the field of electronics and laid the foundation for modern electrical engineering.
35. How did Thomson's experiment relate to the concept of subatomic particles?
Thomson's experiment introduced the concept of subatomic particles to the scientific community. By discovering the electron, he showed that atoms were not the smallest units of matter, but were composed of even smaller particles. This opened up a new field of study into subatomic particles and laid the groundwork for the discovery of other particles like protons and neutrons.
36. What was the significance of Thomson's discovery in terms of chemical bonding?
Thomson's discovery of the electron was fundamental to understanding chemical bonding. It led to the realization that electrons play a crucial role in holding atoms together in molecules. This paved the way for theories of covalent bonding, where atoms share electrons, and ionic bonding, where electrons are transferred between atoms.
37. What was the importance of Thomson's work in the context of the periodic table?
Thomson's discovery of the electron helped explain some of the periodic trends observed in the periodic table. The concept of electrons as fundamental components of all atoms provided a basis for understanding chemical properties and reactivity. It also set the stage for later work on electron configurations, which would explain the periodicity of elemental properties.
38. How did Thomson's experiment contribute to the development of television technology?
Thomson's work on cathode rays was fundamental to the development of television technology. The cathode ray tube he used in his experiments was a precursor to the tubes used in early televisions. His understanding of how electrons could be deflected by electric and magnetic fields was crucial for developing the technology to control electron beams in TV screens.
39. What was the relationship between Thomson's experiment and the concept of elementary particles?
Thomson's discovery of the electron marked the beginning of elementary particle physics. The electron was the first subatomic particle to be identified, and it demonstrated that atoms were not elementary particles themselves. This opened up a whole new field of study into the fundamental building blocks of matter, leading to the discovery of many other elementary particles.
40. How did Thomson's experiment relate to the concept of electromagnetic waves?
While Thomson's experiment showed that cathode rays were particles (electrons) and not electromagnetic waves, it didn't contradict the existence of electromagnetic waves. Instead, it helped clarify the distinction between particles and waves in physics. This work contributed to the later development of wave-particle duality in quantum mechanics.
41. What was the significance of Thomson's experiment in the context of radioactivity studies?
Although Thomson's experiment didn't directly involve radioactivity, it was crucial for understanding radioactive phenomena. The discovery of the electron provided a framework for interpreting beta radiation, which was later identified as high-energy electrons emitted during radioactive decay. Thomson's work thus contributed to the early understanding of radioactive processes.
42. How did Thomson's experiment influence the development of atomic clocks?
Thomson's discovery of the electron and his methods for manipulating charged particles with electromagnetic fields laid the groundwork for many later technologies, including atomic clocks. While not directly related, the principles of electron behavior in electromagnetic fields are crucial in the operation of atomic clocks, which use the energy states of electrons in atoms to measure time with extreme precision.
43. What was the significance of Thomson's experiment in the development of particle accelerators?
Thomson's experiment was a precursor to modern particle accelerators. His method of accelerating electrons using an electric field and manipulating their path with magnetic fields is the basic principle behind all particle accelerators. This work was crucial in the development of more advanced accelerators used in particle physics research.
44. How did Thomson's experiment relate to the concept of electron shells?
While Thomson's experiment didn't directly lead to the concept of electron shells, it was a necessary first step. By discovering the electron, Thomson opened the door to questions about how electrons were arranged in atoms. This led to later work by scientists like Bohr and others, who developed models of electron shells to explain atomic spectra and chemical properties.
45. What was the importance of Thomson's experiment in understanding electrical resistance?
Thomson's discovery of the electron provided a fundamental explanation for electrical resistance. It showed that resistance occurs due to the collision of flowing electrons with the atoms of the conductor. This understanding was crucial for the development of electrical engineering and the design of electronic components.
46. How did Thomson's experiment contribute to the field of spectroscopy?
Thomson's work on cathode rays and the discovery of the electron were crucial for understanding atomic spectra. While he didn't directly work on spectroscopy, his findings about electrons laid the groundwork for later theories explaining how electrons in atoms produce characteristic spectral lines when excited, which is the basis of spectroscopy.
47. What was the significance of Thomson's experiment in the context of nuclear physics?
Although Thomson's experiment didn't directly involve the atomic nucleus (which hadn't been discovered yet), it was a crucial step towards nuclear physics. By showing that atoms had internal structure and contained negatively charged particles, it set the stage for the later discovery of the nucleus and the development of nuclear physics.
48. How did Thomson's experiment relate to the concept of valence electrons?
Thomson's discovery of the electron was fundamental to the development of valence theory. While he didn't propose the concept of valence electrons himself, his work showed that atoms contained electrons, which led to later theories about how electrons participate in chemical bonding. This ultimately resulted in the concept of valence electrons as the key to understanding chemical reactivity.
49. What was the importance of Thomson's experiment in the development of vacuum tube technology?
Thomson's cathode ray experiment was pivotal in the development of vacuum tube technology. His work demonstrated how electrons could be emitted, accelerated, and controlled within a vacuum, which are the basic principles of vacuum tubes. This led to the development of various types of vacuum tubes used in early electronics, including amplifiers and early computer components.
50. How did Thomson's experiment contribute to the understanding of thermionic emission?
Thomson's experiment utilized thermionic emission, although he didn't fully understand the process at the time. The heated cathode in his tube emitted electrons through this process. His work laid the foundation for further studies on thermionic emission, which became crucial in the development of various electronic devices, including vacuum tubes and cathode ray tubes.
51. What was the significance of Thomson's experiment in terms of understanding atomic number?
While Thomson's experiment didn't directly lead to the concept of atomic number, it was an important step in that direction. By discovering the electron, Thomson showed that atoms had internal structure. This led to questions about how many electrons each atom contained, which eventually contributed to the development of the concept of atomic number by later scientists.
52. How did Thomson's experiment relate to the photoelectric effect?
Thomson's discovery of the electron was crucial for understanding the photoelectric effect, although he didn't work on it directly. When Einstein later explained the photoelectric effect in terms of light causing electrons to be emitted from a metal surface, he was building on the foundation of electron theory established by Thomson's work.
53. What was the importance of Thomson's experiment in the context of quantum mechanics?
While Thomson's work predated quantum mechanics, his discovery of the electron was a crucial step towards its development. The electron became one of the first subjects of quantum mechanical treatment, with its wave-like properties being explained by de Broglie and Schrödinger. Thomson's work thus laid the groundwork for many key concepts in quantum mechanics.
54. How did Thomson's experiment contribute to the understanding of electron affinity?
Thomson's discovery of the electron was fundamental to the concept of electron affinity. While he didn't work on electron affinity directly, his demonstration that atoms contained removable negative charges (electrons) was crucial for later understanding of how atoms can gain or lose electrons. This led to the development of concepts like electron affinity and ionization energy.
55. What was the significance of Thomson's experiment in the development of electron microscopy?
Thomson's work on cathode rays and the manipulation of electron beams was a crucial precursor to electron microscopy. While he didn't develop the electron microscope himself, his methods for controlling electron beams with electromagnetic fields laid the groundwork for later scientists to develop electron microscopes, which use beams of electrons to create images at much higher magnifications than optical microscopes.
56. How did Thomson's experiment relate to the concept of electron spin?
Thomson's experiment didn't directly address electron spin, which was discovered much later. However, his discovery of the electron was a necessary first step. The concept of electron spin emerged from later experiments that showed electrons had additional properties beyond charge and mass, properties that couldn't be explained by classical physics. Thomson's work thus set the stage for these later discoveries.