Heat Transfer - Thermal, Conduction, Convection, Radiation, FAQs
In this article we will discuss thermal conduction, examples of conduction, heat transfer, conduction heat, radiant heat transfer, conduction heat transfer, heat energy examples, convective heat, heat conduction examples, transmission of heat, thermal conduction examples, heat transfer examples, conduction heat transfer examples, transfer of heat in solids, heat transfer in fluids, heat energy transfer, principles of heat transfer, heat transfer from hot to cold, heat transfer thermodynamics, and conduction of heat examples.
This Story also Contains
- Heat transfer
- Thermal Conduction
- Thermal Convection
- Thermal Radiation
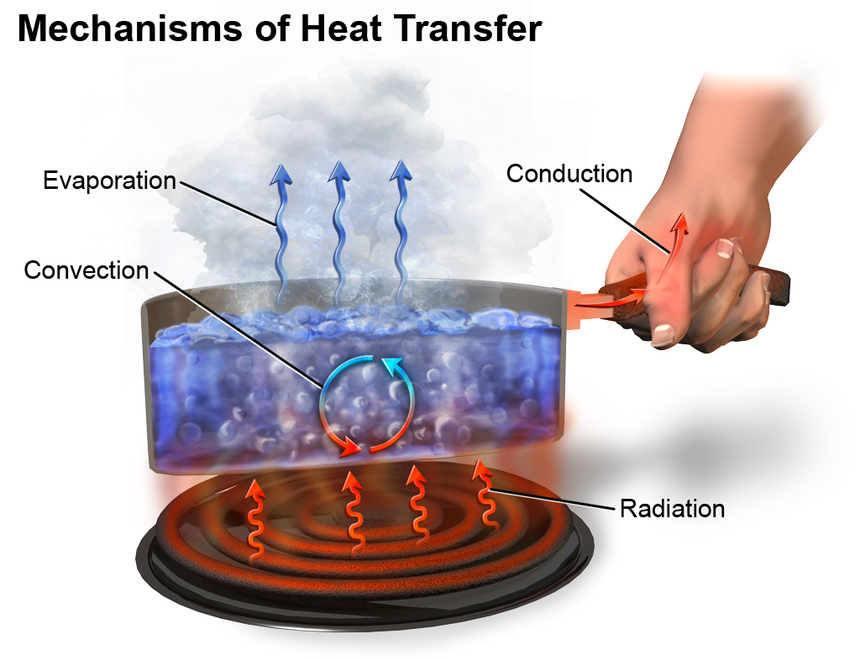
Heat transfer
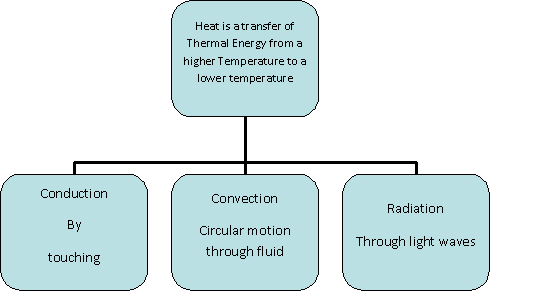
Heat transfer thermodynamics is a discipline of physical sciences related to the exchange, formation, application and conversion of thermal energy known as heat energy between systems. Heat can be transferred by various methods such as thermal conduction, thermal radiation, and thermal convection and sometimes by phase change.
Also read -
Thermal Conduction
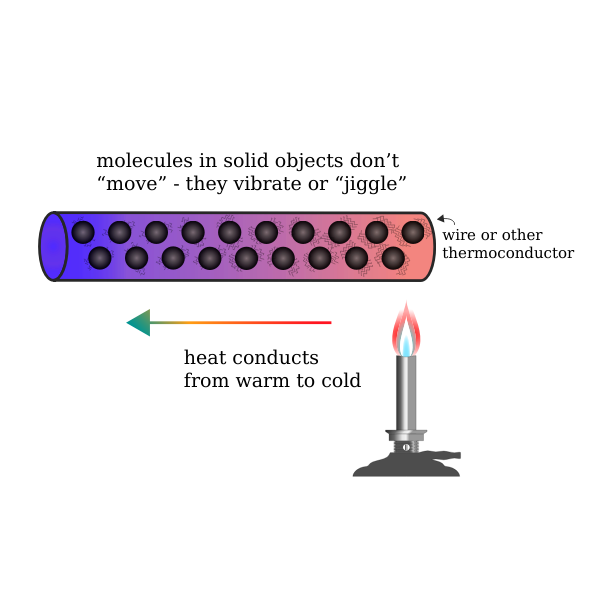
Thermal conduction involves the transfer of internal energy which takes place by the collision of particles on a microscopic level and electron movement in a system. The internal energy is a combination of the kinetic and potential energy of colliding particles which could be atoms, molecules, electrons, etc. thermal conduction is not limited to any particular phase. In other words, thermal conduction of heat transfer in fluids, as well as the transfer of heat in solids, is also possible.
In thermal conduction, heat flows from one body to another and spreads through the receiving body or part of the system. Microscopically, thermal conduction takes place due to exothermically rapid movement or vibration of colliding particles. When these particles having high heat energy interact with particles of low energy, a portion of them are transferred to these low state particles. In simpler words, thermal conduction occurs when neighbouring particles vibrate against each other. In solid objects, thermal conduction is a significant method of heat transfer.
Transfer of heat in solid takes place when two solid objects come in thermal contact. Heat transfer in fluid (both gases and liquids) by thermal conduction is not effective as fluids; especially gases are relatively less conductive. In thermal conduction, heat transfer from one body to another takes place without the displacement of particles. This could be observed when heat is transferred from hot to cold bodies.
Examples of heat transfer from hot to cold bodies could be easily identified in our day to day lives. For example, thermal conduction occurs from the warm skin of the hand when we touch a cold doorknob. But this doesn’t happen when we hold our hand close to the knob without touching it. This is due to poor thermal conduction of air whereas a solid steel knob is a better conductor of heat.
Q= [K.ATh-Tc]/d
Related Topics Link, |
Thermal Convection
When thermal energy expands the fluid, buoyancy forces induce the flow of fluid. This flow of fluid results in the transfer of energy. This process is often called convection.
Thermal convection is a process in which the transfer of heat from one system to another takes place by the movement of fluids. Transfer of heat in convection occurs via mass transfer. Heat transfer by convection is enhanced due to the bulk motion of solids. For example, heat transfer between fluid and solid surface. Thermal convection is the dominant method of heat transfer in liquids and gases.
In systems like streams and currents where bulk fluid motion is observed, free or natural convection occurs. These bulk fluid motions are generated due to buoyancy caused by density variations which are a result of temperature variation in the fluid.
When an external source like fans, pumps, stirrers, etc., are used to induce artificial convection currents such convection is known as forced convection.
Q = hc*A*(Ts-Tf)
Also, students can refer,
Thermal Radiation
In matter, the thermal motion of particles generates electromagnetic radiation. This radiation is termed as thermal radiation. When charges in the material move, heat is generated. This heat is converted into electromagnetic radiation. Thermal radiation involves the conversion of thermal energy into electromagnetic energy. Thermal energy is the kinetic energy generated when the movement of atoms or molecules in matter takes place. All matter has a temperature greater than absolute 0 emits thermal radiation. An example of thermal radiation is infrared radiation emitted by animals. When the physical characteristics of a radiating body resemble the physical characteristics of a black body, the radiation is said to be black body radiation.
When a body emits thermal radiation at any temperature, it consists of a wide range of frequencies. As the temperature of the emitter increases, the dominant frequency range shifts to a higher frequency. For example, a red hot object emits radiation in the low-frequency range of the visible band thus appearing red-orange. All properties of radiation such as polarization, coherence, and wavelength follow the principle of reciprocity which states that the rate of emission of electromagnetic radiation at a given frequency is directly proportional to the amount of absorption. That means if a substance absorbs more blue light then it will radiate thermally more blue light.
P=e*σ*A*(Tr-Tc)4
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: