Lewis Electron Dot Structures
Lewis Electron Dot Structures, colloquially known as Lewis structures, are significant to chemistry because they provide a diagram to indicate the valence electrons of atoms and molecules. Proposed by Gilbert N. Lewis in 1916, such diagrams have been proven to be quite simple for comprehending the nature of compound formation due to atomic bonding. The Lewis structures depict an electron where one dot represents one valence electron and the lines connecting the atoms represent one shared pair of electrons, which are also referred to as one covalent bond. This representation forms the basis for knowing bonding in chemicals, the geometrical shapes of molecules, and the reactivity of substances.
This Story also Contains
- Lewis Electron Dot Structures
- Lewis Symbols of Elements
- Lewis Structures
- Types and Aspects of Lewis Structures
- The key bonding types include:
- Importance and Uses of Lewis Structures
- Some Solved Examples
Also Read -
Lewis Electron Dot Structures
Lewis electron structures are a simple diagram that shows the valence electrons in an atom or molecule and shows where the electrons are located and how they potentially bond or share with other atoms. In a nutshell, it is just an accepted fact that atoms will go to bond in a manner that tends to achieve stable electron configurations, effects that of noble gases or eight electrons in their outer shell.
A Lewis structure is nothing more than a chemical symbol for an element with dots equal to the number of valence electrons it contains. For example, the six valence electrons of oxygen would be set around the oxygen symbol as six dots. When bonding, these dots can be paired to represent shared electrons. This is a tool that helps in interpreting the kind of chemical bond; single, double, or triple bond and that helps to learn a molecular structure and the reactivity of the chemical compound.
Lewis Symbols of Elements
The number of electrons present in the outermost shell is known as valence electrons. For example, the electronic configuration of sodium (Na) is 2, 8, 1, thus, sodium has one valence electron. According to the long form of the periodic table, in the case of representative elements, the group number is equal to the number of valence electrons. The valence electrons in atoms are shown in terms of Lewis symbols. To write the Lewis symbol for an element, we write down its symbol surrounded by several dots or crosses equal to the number of valence electrons. Paired and unpaired valence electrons are also indicated. The Lewis symbols for some of the important elements are shown below:

Lewis Structures
We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:

The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding also known as lone pairs of electrons and one shared pair of electrons. A dash (or line) is sometimes used to indicate a shared pair of electrons:

A single shared pair of electrons is called a single bond. Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond.
To draw the Lewis structure for any molecule like CO, we follow the following five steps:
-
Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 4 (from C) + 6 (from O) = 10.
-
Draw a skeleton structure of the molecule. We can easily draw a skeleton with a C–O single bond:
C–O
-
Distribute the remaining electrons as lone pairs on the terminal atoms. In this case, there is no central atom, so we distribute the electrons around both atoms. We give eight electrons to the more electronegative atom in these situations; thus oxygen has the filled valence shell:

-
Place all remaining electrons on the central atom. Since there are no remaining electrons, this step does not apply.
-
Rearrange the electrons to make multiple bonds with the central atom in order to obtain octets wherever possible. In this case, carbon has only four electrons around it. To move to an octet for carbon, we take two of the lone pairs from oxygen and use it to form a CO triple bond.

This satisfies the Octet condition for both atoms.
Types and Aspects of Lewis Structures
Lewis structures can be classified according to the bonding types being represented.
The key bonding types include:
Single Bonds: This is a single in-line connecting between two atoms showing that only one electron pair is covalently bonded.
Double Bonds: Represented by two lines. This indicates that between atoms, two pairs of electrons are shared.
Triple Bonds: Represented by three lines. This indicates that between atoms, three pairs of electrons are shared.
Besides the bond types above, there are also lone pairs of electrons which are reported to be nonbonding electrons found in one of the atoms. For example in water, H₂O the Lewis structure involves two single bonds between the oxygen and hydrogen atoms along with two pairs of lone electrons on the oxygen.
By their very definition, Lewis structures can also be extended to be drawn for polyatomic ions as the total of electrons is added or removed as per the charge on the ion. When the example of the sulfate ion, SO₄²⁻ is considered, the additional electrons in the Lewis structure are represented because two extra electrons are added. This is due to the fact reported above: the Lewis structures are thus versatile and provide the chemist with a powerful way of representing the compound.
Also Check-
Importance and Uses of Lewis Structures
Lewis Electron Dot Structures are used in many applications other than just for classroom teaching and many uses in pharmaceuticals, materials science, and environmental chemistry. Lewis structures are used by chemists in designing a new drug around predictions concerning how the molecules are going to interact with the biological system. That is, able to modify compounds if an understanding of the arrangement of electrons ensures they will be more potent or, in turn, have fewer side effects. Beyond inorganic chemistry, Lewis structures also help in the refinement or compositional elucidation of new materials of dual-phase, mixed-matrix, and other forms, which have some kind of electrically conducting or mechanically strong desired properties. For example, the design of many polymers starts with the intimate knowledge of the bonding among constituent monomers and their electron distribution within the monomer.
Related Topics:
Recommended topic video on (Lewis Electron dot structure)
Also Read:
Some Solved Examples
Example 1:
What is the total number of lone pair of electrons in I3- ion?
1) 9
2) 3
3) 6
4) 12
Solution:
Lewis structure -
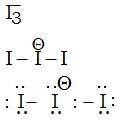
No. of lone pairs =9
Hence, option number (1) is correct.
Example 2:
How many bond pair electrons and lone pair electrons are present in NO3-1?
1) 6,18
2) 6,16
3) 8,18
4) 8,16
Solution:
The Structure of NO3-1
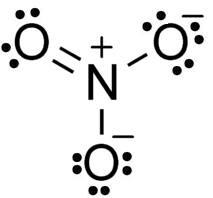
In this structure, lone pairs of electrons are used to complete the central atom octet by forming the double bond as shown in the figure.
Therefore 8 bond pairs of electrons and 16 lone pairs of electrons are present in NO3-1.
Hence, option number (4) is correct.
Example 3:
Which is the correct electron dot structure of N2O ?
1)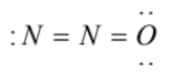

2) 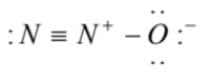

3)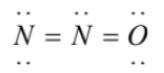

4)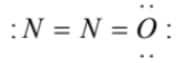

Solution:
(1) 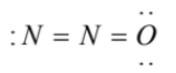 has an incomplete octet on terminal nitrogen
has an incomplete octet on terminal nitrogen
(2) 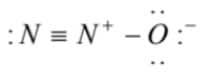 is the correct lewis dot structure
is the correct lewis dot structure
(3) 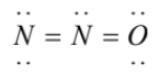 has an overcomplete octet on central nitrogen
has an overcomplete octet on central nitrogen
(4) 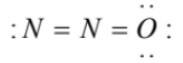 has an incomplete octet on terminal nitrogen and overcomplete octet on the oxygen atom
has an incomplete octet on terminal nitrogen and overcomplete octet on the oxygen atom
Hence, only the structure given in Option (2) is correct
Example 4:
What is the nature of the bond between B and O in $\mathrm{C}_2 \mathrm{H}_5 \mathrm{OBH}_3$?
1) Covalent
2 ) CO-ordinate bond
3) Ionic bond
4) Banana shape bond
Solution
The type of bond present in $\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{OBH}_3$ is a coordinate covalent bond. The lone pair over the Oxygen is donated to the vacant p orbital of the Boron atom as shown below:
$\left(\mathrm{C}_2 \mathrm{H}_5\right)_2 \mathrm{O}: \rightarrow \mathrm{BH}_3$
Hence, the answer is the option (2).
Practice More Questions From the Link Given Below:
| Lewis Electron Dot Structures | Chemical Bonding |
| Formal Charge And Its Properties | Limitations of The Octet Rule |
Conclusion
Lewis electron dot structures, therefore, represent one of the basic concepts in chemistry to be learned, providing the right visual framework with which one may readily understand those valence electrons involved in the bonding interactions between atoms. These structures elaborate the complexity of chemical bonds and state that atoms share electrons to form stable electronic configurations, basically by the octet rule. Using dots to represent valence electrons, the electron configuration becomes a line representing pairs that are shared. The Lewis dot structure provides insight into molecular shape and reactivity, as well as into the nature of the chemical bond—from single to triple.
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
A Lewis dot structure (or Lewis electron‑dot structure) is a diagram showing the valence electrons of atoms in a molecule as dots. Lines (or paired dots) represent shared pairs of electrons (i.e. bonds), and unshared electrons are shown as lone pairs.
- Count all the valence electrons from each atom (taking into account any charge if molecule is ion).
- Decide which atom goes in the center (usually the least electronegative, hydrogen is never central).
- Connect outer atoms to the central atom using single bonds (each bond = 2 electrons).
- Distribute the remaining electrons to satisfy octets (or duet for hydrogen) starting with outer atoms, then the central atom.
- If the central atom lacks an octet, form double or triple bonds as needed.
- Check formal charges: try to minimise formal charges and place negative formal charges (if any) on more electronegative atoms.
Yes. For ions: you adjust the total valence electron count by adding electrons for negative charges, or subtracting for positive charges. Then you draw structure as usual, and bracket the structure with the charge.
- Once you know how many bonding pairs and lone pairs are on the central atom (from Lewis structure), you apply VSEPR (valence shell electron pair repulsion) theory to predict shape / geometry.
- Also, by seeing which atoms have lone pairs, or what kinds of bonds (polar bonds) are there, you can infer if the molecule is polar or nonpolar.
- Once you know how many bonding pairs and lone pairs are on the central atom (from Lewis structure), you apply VSEPR (valence shell electron pair repulsion) theory to predict shape / geometry.
- Also, by seeing which atoms have lone pairs, or what kinds of bonds (polar bonds) are there, you can infer if the molecule is polar or nonpolar.
Yes. For ions: you adjust the total valence electron count by adding electrons for negative charges, or subtracting for positive charges. Then you draw structure as usual, and bracket the structure with the charge.
- Count all the valence electrons from each atom (taking into account any charge if molecule is ion).
- Decide which atom goes in the center (usually the least electronegative, hydrogen is never central).
- Connect outer atoms to the central atom using single bonds (each bond = 2 electrons).
- Distribute the remaining electrons to satisfy octets (or duet for hydrogen) starting with outer atoms, then the central atom.
- If the central atom lacks an octet, form double or triple bonds as needed.
- Check formal charges: try to minimise formal charges and place negative formal charges (if any) on more electronegative atoms.
- To show how atoms bond in a molecule (who shares electrons with whom).
- To visualize lone pairs of electrons (those not involved in bonding).
- To help predict molecular shapes (with VSEPR), reactivity, and formal charges.
A Lewis dot structure (or Lewis electron‑dot structure) is a diagram showing the valence electrons of atoms in a molecule as dots. Lines (or paired dots) represent shared pairs of electrons (i.e. bonds), and unshared electrons are shown as lone pairs.
- To show how atoms bond in a molecule (who shares electrons with whom).
- To visualize lone pairs of electrons (those not involved in bonding).
- To help predict molecular shapes (with VSEPR), reactivity, and formal charges.