Dragos Rule
How do we predict the stability and geometry of covalent hydrides? Why are some hydrides stable and while others are unstable and decompose easily? We will get these answers by studying Drago's rule. Drago’s Rule helps in understanding the bonding in hydrides of Group 13 and 14 elements, particularly in predicting whether $d-p \pi$ bonding or back-donation will occur or not.

Drago's rule is an empirically based concept applied with an understanding of bond angles and molecular geometries of some hydrides; particularly, those that contain the elements of groups 15 and 16 from the periodic table are considered. This is admitted to include elements in the third period and lower, like phosphorus, arsenic, antimony, sulfur, selenium, and tellurium.
Drago's rule
Drago’s Rule is a principle in inorganic chemistry used to predict the bonding behavior of hydrides, particularly of Group 13 (B, Al, Ga) and Group 14 (C, Si, Ge, etc.) elements. Dragos rule explains the bond angles of hydrides of groups 14, 15, and 16 and 2nd members of each of these three groups.
According to Drago's rule, if the central atom is less electronegative and has a low tendency to form multiple bonds, and the hydrogen atoms are not strongly electronegative, then the hybridization of the central atom is not necessary for bond formation. Instead, the bonds are formed using pure atomic orbitals without mixing.
In Drago’s rule, when the various conditions are satisfied as mentioned below, then the energy difference will be very high between the participating atomic orbitals, and hence no mixing of orbitals or hybridization takes place.
-
At least one lone pair must be present on the central atom.
-
The central atom must be off or below the 3rd period.
-
The electronegativity of the surrounding atoms must be less than or equal to 2.1.
-
For these hydrides, hybridization does not take place, and thus bonding takes place only through pure atomic p orbitals, like in PH3, and hence the bond angle will be approximately $90^0$.
For example, the bond angle for $\mathrm{H}_2 \mathrm{O}$ is 104.50 but for $\mathrm{S}_2 \mathrm{H}, \mathrm{Se}_2 \mathrm{H}$, and $\mathrm{Te}_2 \mathrm{H}$, the bond angles are approximately $90^0$.
Conditions and Implications of the Drago Rule
The following must be met for Drago's rule to be valid in a good number of cases:
1. Lone Pairs: The central atom has to contain a lone pair of electrons.
2. Element Group: It has to be a member of groups 13 through 16, be on the third period, and be on.
3. Electronegativity: The centrality of the atom, concerning electronegativity, should be 2.5 or less.
In such cases, the sum of sigma bonds and lone pairs on the central atom becomes four. Hybridization is hence not required, and atomic orbitals may directly participate in bond formation. This leads to odd molecular geometries that do not ideally fall into conventional theories of hybridization.
Bond Angle
Bond angle is the angle between two bonds that form between two atoms. The figure given below illustrates the concept.

Real-Life Applications and Relativity
Drago's rule has a huge impact on describing the behavior of so many molecules in such diverse areas. Take for instance the compound phosphine, PH3. The central phosphorus atom contains a lone pair of electrons. As we all have an idea, it falls under group 15 in the periodic table. In addition, its electronegativity is 2.19. Applying Drago's rule, it could easily be derived that the s-character percent in P-H bonds is less, about 6%. Hence, the lone pair in the compound remains in an orbital that is highly rich in s-character. This is something that never happens in a hybridized orbital. This causes the bond angle to be about 90°, which again deviates from the idealized geometry of a tetrahedron.
Recommended topic video on (Bond angle and Drago's rule)
Some Solved Examples
Example 1: Why is NH3 a stronger Lewis base than PH3?
1) (correct)In NH3 lone pair is present in one of the sp3 hybridized orbitals, while in PH3 lone pair is present in pure s-orbital.
2)In NH3 lone pair is present in a pure s-orbital, while in PH3 lone pair is present in one of the sp3 hybridized orbitals.
3)In NH3 lone pair is present in one of the sp3d hybridised orbitals, while in PH3 lone pair is present in pure s-orbital
4)None
Solution
In NH3 lone pair is present in one of the sp3 hybridized orbitals, while in PH3 lone pair is present in pure s-orbital.
Hence, the lone pair donation capacity of NH3 is stronger than PH3.
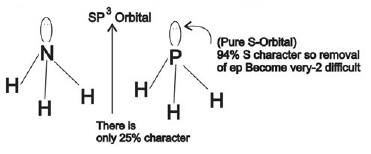
Therefore, option number(1) is correct.
Example 2: As the s-character of a hybridized orbital decreases, the bond angle:
1) Decreases (correct)
2) Increases
3) Does not change
4) Becomes zero
Solution
As the s-character of hybridized orbitals decreases, the bond angle also decreases. In sp3 hybridisation: s-character (1/4), bond angle 109°. In sp2 hybridization: s-character (1/3), bond angle 120°. In sp hybridization: s-character (1/2), bond angle 180°.
Hence, the answer is option (1).
Example 3: The HCH bond angle in {HCHO} is nearly equal to:
1) $120^0$ (correct)
2) $90^{\circ}$
3) $60^0$
4) $ 180^0$
Solution
The hybridization of HCHO is sp2
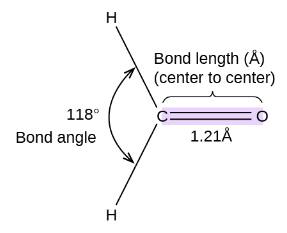
The bond angle in HCHO is close to 120o Facing repulsion by the lone pair of Oxygen.
Hence, option number (1) is correct
Example 4: Which one of the following compounds has the smallest bond angle in its molecule?
1) $\mathrm{SO}_2 \mathrm{SO} 2$
2) $\mathrm{OH}_2 \mathrm{OH} 2$
3) $\left(\mathrm{SH}_2\right)$ (correct) $\mathrm{SH}_2$
4) $\mathrm{NH}_3 \mathrm{NH}-$
Solution:
The repulsion force acts on bonds due to a lone pair present on the central atom, then the bond angle between multiple bonds decreases.
As the number of lone pairs increases bond angle decreases.
As the size of the central atom increases and lone pairs are also present then the bond angle decreases more.
| Molecule | Bond angle | Number of Lone pairs |
| $\mathrm{SO}_2$ | $119.5^0$ | 1 |
| $\mathrm{H}_2 \mathrm{O}$ | $104.5^0$ | 2 |
| $\mathrm{H}_2 \mathrm{S}$ | $92.5^0$ | 2 |
| $\mathrm{NH}_3$ | $107^0$ | 1 |
O and S have two lone pair.
Atom size S > O
So, $\mathrm{H}_2 \mathrm{S}$has the smallest bond angle.
Hence, the answer is the option (3).
Example 5: The dipole moments of CCl4, CHCl3 and CH4 are in the order:
1) (Correct)$\mathrm{CH}_4=\mathrm{CCl}_4<\mathrm{CHCl}_3$$\mathrm{CH}_4=\mathrm{CCl}_4<\mathrm{CHCl}_3$CH4=CCl4<CHCl3
2)$\mathrm{CHCl}_3<\mathrm{CH}_4=\mathrm{CCl}_4$CHCl3<CH4=CCl4
3)$\mathrm{CH}_4<\mathrm{CCl}_4<\mathrm{CHCl}_3$CH4<CCl4<CHCl3
4)$\mathrm{CCl}_4<\mathrm{CH}_4<\mathrm{CHCl}_3$CCl4<CH4<CHCl3
Solution
Let us first look at the structures of the given compounds
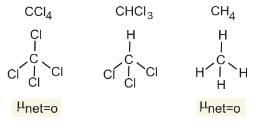
Clearly $\mu \neq 0$ of $\mathrm{CHCl}_3$
Therefore, Option(1) is correct.
$\mathrm{CH}_4=\mathrm{CCl}_4<\mathrm{CHCl}_3$
Practice More Questions with the Link Given Below
Conclusion
In a nutshell, one such principle that aids inorganic chemistry in obtaining knowledge about molecular geometries and bond angles of certain hydride molecules is Drago's rule. Therefore, it can be done that much predictions and explanations of the behavior of these molecules under different contexts by the chemist, based on an understanding of the conditions under which this rule operates and its implications.
Also read