Valence Bond Theory - Explanation, Examples, Applications, Limitations, FAQs
Valence bond theory is very simplistic and forms one of the central factors in chemistry in explaining covalent bond formation between atoms. Heitler and London developed it in 1927 as a method that would explain the stability and directionality of chemical bonds. VBT is based on the idea that covalent bonds arise from the overlap of atomic orbitals, sharing electron pairs between atoms. VBT concerns key parameters such as bond order, resonance, and resonance hybridization. The Valence Bond Theory was developed in order to explain chemical bonding using the method of quantum mechanics.
This Story also Contains
- Bond Parameters
- Resonance and Resonance Hybridization
- Applications and Relevance
- Some Solved Examples
One of the core ideas of VBT, bond order, is essentially the number of shared pairs of electrons between two atoms. The higher the bond order, the stronger and shorter the bond will be.
Ethene, C2H4, is 2, and it is 3 in ethyne. Hence, the bond is stronger and shorter than in ethene. This will provide the basis for explaining the stability and reactivity of molecules and their physical properties, such as melting and boiling points. Another very useful concept in VBT is resonance, which explains the extra stability of some molecules and ions.
Resonance is the concept by which a single molecule can be represented by two or more equivalent Lewis structures. These resonance structures get mixed into an actual structure of the molecule, called resonance hybridization. This hybridization results in electron delocalization, accounting for its stabilization and thus, aromaticity. Resonance is rather more important in explaining the structure and reactivity of aromatic compounds like benzene, and its derivatives.
In this article, we will cover the concept of the Valence Bond Theory. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read :
Bond Parameters
A few important parameters of a covalent bond, which are centrally located in VBT, define the strength and stability of the bond. The basic important parameters of a covalent bond, which are at the heart of VBT, are the following:
Bond order: The number of electron pairs shared by two atoms; it is directly proportional to the bond strength. A higher bond order would hence imply it will be a stronger bond with a shorter length. For example, the carbon-carbon bond of ethene, C2H4, is a double bond with a bond order of 2, whereas that of ethyne, C2H2, is a triple bond with a bond order of 3; hence it is stronger and shorter than that in ethene.
Valence bond theory describes a covalent bond as the overlap of half-filled atomic orbitals (each containing a single electron) that yield a pair of electrons shared between the two bonded atoms. We say that orbitals on two different atoms overlap when a portion of one orbital and a portion of a second orbital occupy the same region of space. According to valence bond theory, a covalent bond results when two conditions are met:
-
An orbital on one atom overlaps an orbital on a second atom.
-
The single electrons in each orbital combine to form an electron pair.
The mutual attraction between these negatively charged electron pairs and the two positively charged nuclei serves to physically link the two atoms through a force we define as a covalent bond. The strength of a covalent bond depends on the extent of overlap of the orbitals involved. Orbitals that overlap extensively form bonds that are stronger than those that have less overlap.
The energy of the system depends on how much the orbitals overlap. The figure given below shows how the sum of the energies of two hydrogen atoms (the colored curve) changes as they approach each other. When the atoms are far apart there is no overlap, and by convention, we set the sum of the energies at zero. As the atoms move together, their orbitals begin to overlap. Each electron begins to feel the attraction of the nucleus in the other atom. In addition, the electrons begin to repel each other, as do the nuclei. While the atoms are still widely separated, the attractions are slightly stronger than the repulsions, and the energy of the system decreases. As the atoms move closer together, the overlap increases, so the attraction of the nuclei for the electrons continues to increase. At some specific distance between the atoms, which varies depending on the atoms involved, the energy reaches its lowest (most stable) value. This optimum distance between the two bonded nuclei is the bond distance between the two atoms. The bond is stable because at this point, the attractive and repulsive forces combine to create the lowest possible energy configuration. If the distance between the nuclei were to decrease further, the repulsion between nuclei and the repulsion between electrons would become stronger than the attractive forces and thus the energy of the system would then rise and make the molecule unstable.

In addition to the distance between two orbitals, the orientation of orbitals also affects their overlap. Greater overlap is possible when orbitals are oriented such that they overlap on a direct line between the two nuclei. The figure given below shows this for two p orbitals from different atoms; the overlap is greater when the orbitals overlap end to end rather than at an angle.

(a) The overlap of two p orbitals is greatest when the orbitals are directed end to end.
(b) Any other arrangement results in less overlap. The dots indicate the locations of the nuclei.
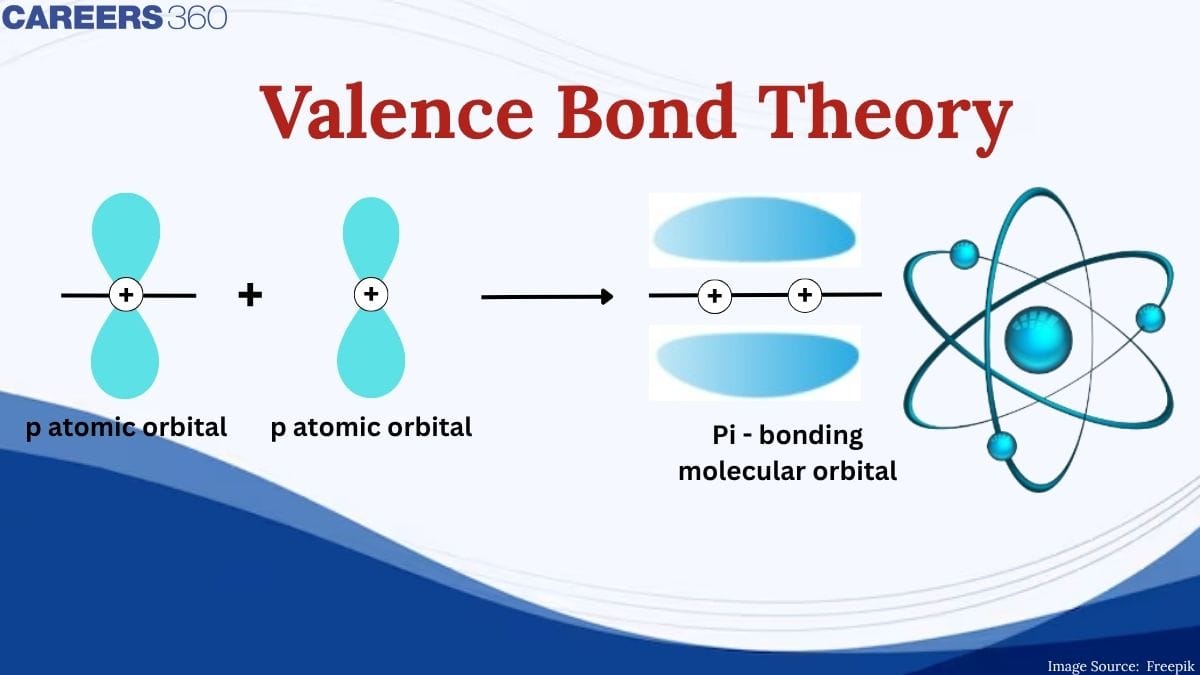
The overlap of two s orbitals, the overlap of an s orbital and a p orbital, and the end-to-end overlap of two p orbitals all produce sigma bonds (σ bonds), as given in the figure below. A σ bond is a covalent bond in which the electron density is concentrated in the region along the internuclear axis; that is, a line between the nuclei would pass through the center of the overlap region. Single bonds in Lewis structures are described as σ bonds in valence bond theory.

Sigma (σ) bonds form from the overlap of the following: (a) two s orbitals, (b) an s orbital and a p orbital, and (c) two p orbitals.

A pi bond (π bond) is a type of covalent bond that results from the side-by-side overlap of two p orbitals. In a π bond, the regions of orbital overlap lie on opposite sides of the internuclear axis. Along the axis itself, there is a node, that is, a plane with no probability of finding an electron.

Pi (π) bonds form from the side-by-side overlap of two p orbitals.
While all single bonds are σ bonds, multiple bonds consist of both σ and π bonds. As the Lewis structures below suggest, O2 contains a double bond, and N2 contains a triple bond. The double bond consists of one σ bond and one π bond, and the triple bond consists of one σ bond and two π bonds. Between any two atoms, the first bond formed will always be a σ bond, but there can only be one σ bond in any one location. In any multiple bonds, there will be one σ bond, and the remaining one or two bonds will be π bonds.

Also Read :
Resonance and Resonance Hybridization
Resonance is a form in which a molecule can be considered to have two or more equivalent Lewis structures.
This is particularly important in VBT since this explains why some molecules and ions are stable. As one example, two different equivalent Kekulé structures can be drawn for the benzene molecule, in addition to several other resonance structures. All resonance structures contribute to the ground state of benzene, though the actual structure of benzene is a hybrid of its resonance structures, known as resonance hybridization. In this hybridization, delocalization of electrons takes place, hence the stability and aromaticity of the molecule.
Also Check:
Applications and Relevance
The tenets of VBT relate to a very broad spectrum of features in chemistry and beyond.
Within organic chemistry, the VBT aids in understanding the stability and reactivity of molecules: the two most important ingredients in grasping reaction mechanisms and in the design of new compounds. In the case of biochemistry, VBT accounts for most of the structures and functions of the biomolecules, including proteins and nucleic acids. This is clearly accounted for in the example of DNA, which in a double-helix structure is stabilized by hydrogen bonds between complementary base pairs that might otherwise be understood using VBT.
Application of VBT in solid-state chemistry establishes the bonding in crystalline solids and hence opens a window into their physical and chemical properties. VBT also forms the base for other more advanced theories of chemical bonding, for instance, Molecular Orbital Theory, which can give a much fuller description of the way bonding in molecules comes about.
NCERT Chemistry Notes:
Recommended topic video on (Valence Bond Theory)
Some Solved Examples
Example 1:
Determining Bond Order. Calculate the bond order between the two nitrogen atoms in the N2 molecule.
Solution:
To determine the bond order in the N2 molecule, we need to consider the number of shared electron pairs between the nitrogen atoms. In the ground state configuration of the N2 molecule, the molecular orbital diagram shows that there are 10 electrons in the bonding orbitals and 4 electrons in the antibonding orbitals. The bond order can be calculated using the formula:
Bond order = (Number of bonding electrons - Number of antibonding electrons) / 2
Substituting the values:
Bond order = (10 - 4) / 2 = 3
Therefore, the bond order between the two nitrogen atoms in the N2 molecule is 3, indicating a triple bond.
Example 2:
Resonance Structures of Benzene. Draw the resonance structures of benzene (C6H6) and explain their significance.
Solution:
Benzene (C6H6) is a planar aromatic compound with alternating carbon-carbon single and double bonds. However, the actual structure of benzene is a hybrid of multiple resonance structures, which can be represented as follows:
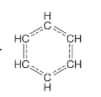
This structure is known as the Kekulé structure of benzene. However, there are two equivalent Kekulé structures possible for benzene, as shown below:
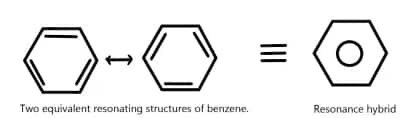
The significance of resonance structures lies in their ability to explain the stability and aromaticity of benzene. The delocalization of electrons in benzene leads to a more stable configuration compared to a structure with localized double bonds.
Example 3:
Resonance Hybridization in Nitrite Ion. Explain the resonance hybridization in the nitrite ion (NO2-).
Solution:
The nitrite ion (NO2-) can be represented by two resonance structures, as shown below:
.jpg)
In the first resonance structure, the nitrogen atom is bonded to one oxygen atom with a double bond and to the other oxygen atom with a single bond. In the second resonance structure, the positions of the double and single bonds are reversed.
The actual structure of the nitrite ion is a resonance hybrid of these two structures, where the nitrogen-oxygen bonds have a partial double bond character. This resonance hybridization results in the delocalization of electrons and contributes to the stability of the nitrite ion.
Example 4:
Bond Order in Ozone. Calculate the bond order of the ozone molecule (O3).
Solution:
Ozone (O3) has two resonance structures that can be represented as follows:
.jpg)
In both resonance structures, one of the oxygen-oxygen bonds is a double bond, while the other is a single bond. The total number of bonding electrons is 6 (from the double bond) and 2 (from the single bond), while there are no antibonding electrons.
Using the bond order formula:
Bond order = (Number of bonding electrons - Number of antibonding electrons) / 2
Bond order = (6 - 0) / 2 = 3
Since there are three bonds (considering the resonance), the bond order for ozone is 1.5, indicating that the bonds between the oxygen atoms are equivalent and exhibit partial double bond character.
Example 5:
Stability of the Acetate Ion. Describe the resonance structures of the acetate ion (C2H3O2-) and their significance.
Solution:
The acetate ion (C2H3O2-) can be represented by two resonance structures, which can be depicted as follows:
.jpg)
In the first resonance structure, the carbon atom is bonded to one oxygen atom with a double bond and to the other oxygen atom with a single bond. In the second resonance structure, the positions of the double and single bonds are reversed.
The actual structure of the acetate ion is a resonance hybrid of these two structures, where the carbon-oxygen bonds have a partial double bond character. This resonance hybridization leads to increased stability of the acetate ion, as the delocalization of electrons reduces the overall energy of the ion.
The significance of these resonance structures is that they explain the equivalency of the two carbon-oxygen bonds in the acetate ion, which have bond lengths that are intermediate between a single and a double bond. This concept is crucial in understanding the reactivity and stability of the acetate ion in various chemical reactions.
Example 6:
Explain the nature of bonding in [Ni(CN)4]2- on the basis of valence bond theory.
Solution:
In the complex [Ni(CN)4]2-, nickel is in +2 oxidation state and has the electronic configuration 3d 8. A cyanide ion delivers a pair of electrons to each of the hybridised orbitals. The ligand cyanide (CN–) is a strong field ligand. Therefore, it will cause pairing of electrons. The hybridisation scheme is as shown in below diagram:
.jpg) The absence of unpaired electrons indicates that the compound is diamagnetic.
The absence of unpaired electrons indicates that the compound is diamagnetic.
Practice More Questions From the Link Given Below:
Related Topics Link:
Summary
VBT, therefore, provides a framework that allows the stability and behavior of chemical species to be appreciated by stressing mainly two parameters such as bond order, resonance, and resonance hybridization. The applications of VBT, therefore, range very broadly from organic synthesis to materials science. So, the research work eventually acquires central value for a very broad range from organic synthesis up to material science applications. The deeper one presses into the details of the molecular world, the more he finds VBT at the center, so to speak, of going about the mission of unraveling the mysteries of chemical bonding, along with its implications for the real world. The Valence Bond Theory is one of the key pillars around which theories for bonding in chemistry were put together to allow for the explanation of the formation of covalent bonding and eventually the properties arising out of such bonds.
Thus, VBT specifies some crucial parameters related to bond order, resonance, and resonance hybridization; it is, therefore, helpful in giving some insight into stability and behavior within chemical species. Some applications of VBT go far beyond just the simple calculation of some molecular properties. With the complex molecular structures and reactivity that emerged, VBT remained crucial for a chemist or a researcher. It is hence in respect of this that one finds relevance and continuous development, underpinning the rooting of this basic principle in the ever-evolving world of chemistry.
Also Check:
Frequently Asked Questions (FAQs)
The “father” (or one of the founders) of Valence Bond Theory is generally considered to be Walter Heitler, together with Fritz London—they first formulated a quantum mechanical treatment of the chemical bond (Heitler‑London theory)
Orbital Overlap
A covalent bond is formed when two atomic orbitals—each containing an unpaired electron—overlap in space. The extent of overlap determines the strength of the bond.Directional Nature
Because bonding depends on how orbitals overlap, the bond has directionality. The orientation of atomic orbitals involved affects the geometry of the molecule.Spin Pairing of Electrons
Only unpaired electrons from the overlapping orbitals can form a bond, and these electrons must have opposite spins.
Valence Bond Theory is a model in chemistry that explains how covalent bonds form between atoms. According to VBT, a covalent bond arises when atomic orbitals from two atoms overlap, and their unpaired electrons pair up in the overlapping region. The shared electrons are thus localized between the two nuclei. The stronger the overlap, the stronger the bond.
Postulates / Main Assumptions of VBT
A covalent bond is formed when two half‑filled atomic orbitals (each carrying one unpaired electron) overlap.
The unpaired electrons in the overlapping orbitals must have opposite spins (i.e. spin pairing).
The overlapping orbitals should have similar energies for effective overlap.
When overlap happens, the electron density in the region between the two nuclei increases, reducing repulsion and stabilizing the system.
A bond forms at an equilibrium distance when potential energy is minimized and the system is most stable.
The strength of the bond depends on the extent of overlap: greater overlap yields stronger bonds.
Bonds are directional—in VBT, the specific orientation of the overlapping orbitals influences molecular geometry.
VBT distinguishes sigma (σ) and pi (π) bonds: σ bonds from head‑on (axial) overlap, π bonds from side‑by‑side overlap.
Electrons in overlapping orbitals remain localized between the two atoms, retaining some atomic orbital character.
It is a concept that describes the interaction of chemicals. VBT states that a complete atomic orbital spacecraft leads to the formation of a chemical bond between two atoms. Unpaid electrons are distributed and an orbital hybrid is formed.
The concept of valence bond fails to explain the tendency of carbon and fails to provide insight into the energy associated with electrons. This theory also assumes that electrons are found in certain areas.
The state of the high fragmentation defined by VBT can be used to describe how cohesive bonds are formed across multiple molecules. The theory may also provide insight into the ionic character of chemical bonds.
Sigma bonds are formed from the splitting of the head to the head of the atomic orbitals that participate in the bond. Pi, on the other hand, bonds involve the same number of atomic orbitals.
Linus Pauling
In the concept of valence bond (VB), highly recommended by American scientists Linus Pauling and John C.