NMR Spectroscopy - Meaning, Principle, Applications, FAQs
Spectroscopy meaning: Nuclear magnetic resonance (NMR) is a spectroscopic technique which is based on the absorption of electromagnetic radiation in the region of 4-900MHz which is referred to as the radiofrequency region, by the nuclei of atoms. Proton nuclear magnetic resonance spectroscopy is one of the important as well as powerful tools which is used for determining the number of proton or hydrogen atoms in the compound.
This Story also Contains
- Basic NMR spectroscopy principle:
- NMR instrumentation
- Shielding and deshielding of protons
- General applications of NMR spectroscopy
It is used to study a wide variety of nuclei which includes 1H, 15N, 19F, 13C,31P, etc. Basically, the nuclei that have an odd number of protons or an odd number of neutrons (or both) have a property called spin which allows them to be studied by NMR i.e., nuclei with an odd mass are NMR active. A basic conclusion can be drawn from this as per the following table:
| Spin Quantum number "(l) | Atomic mass | Atomic number | Examples | NMR (active/inactive) |
Half-integer | Odd | Odd | 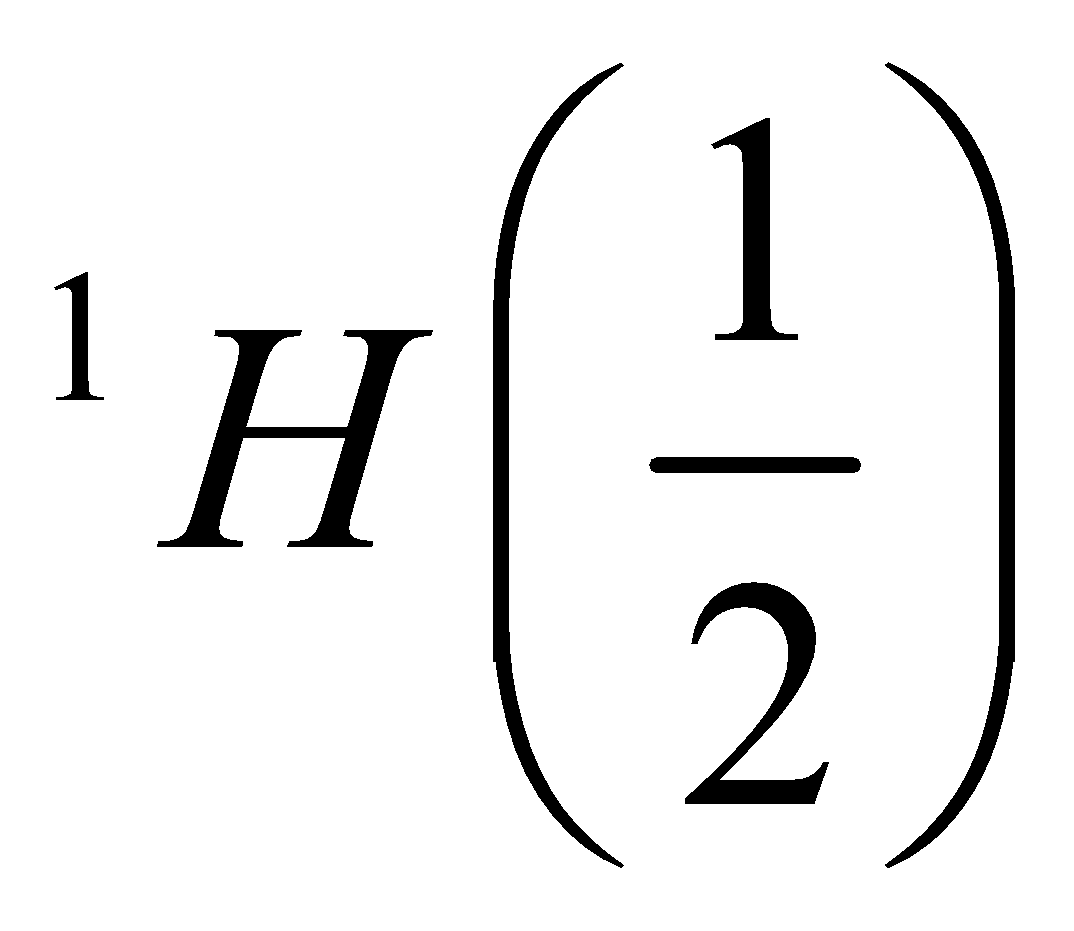 | NMR active |
| Half-integer | Odd | Even | 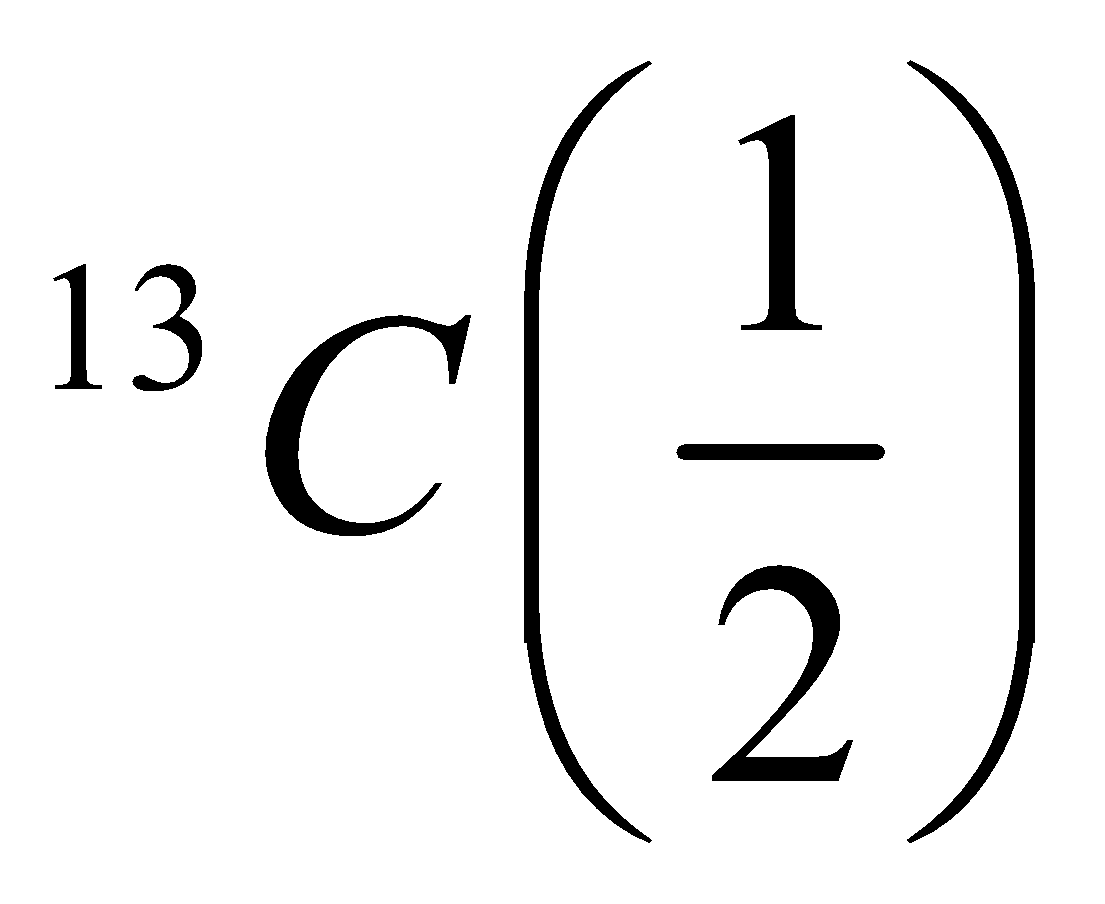 | NMR active |
| Integer | Even | Odd | NMR active | |
| Zero | Even | Even | NMR inactive |
Also read -
Basic NMR spectroscopy principle:
The theory behind NMR comes from the spin of the nucleus of an atom and it generates a magnetic field. Without an external applied magnetic field, the nuclear spins of the atoms are aligned in random directions as shown in the figure below:
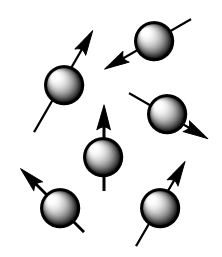
But when an external magnetic field i.e., Bο is present the nuclei align themselves either with or against the field of the external applied magnetic field. The nuclei with magnetic moments which align with the field are lower in energy and are known as α- spin state whereas those with magnetic moments that align against the field are higher in energy and are known as β- spin state. The orientation of spin of is shown as follows:
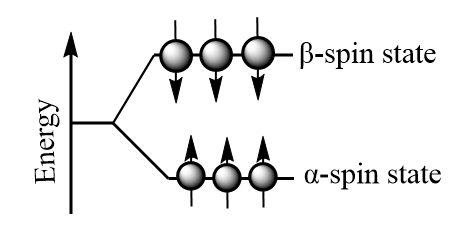
The energy difference ΔE between the α and β spin states depend on the strength of the applied magnetic field (Bο): the greater the strength of the applied magnetic field, the greater the difference in energy. In simple words, the energy difference between the α and β spin states increase as the strength of the applied magnetic field as shown in the diagram below.
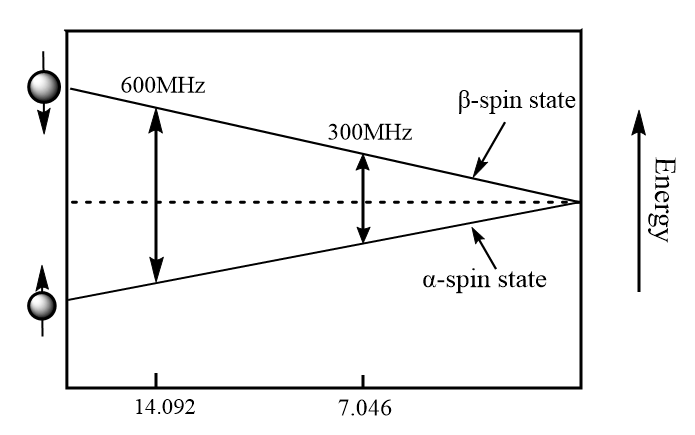
When the external applied magnetic field is removed, the spin returns to its ground state level and the radiofrequency energy is emitted at the same frequency level at which it was absorbed which is responsible to give NMR spectrum of the concerned nucleus. The emitted radiofrequency is directly proportional to strength of applied field and can be expressed mathematically as follows:
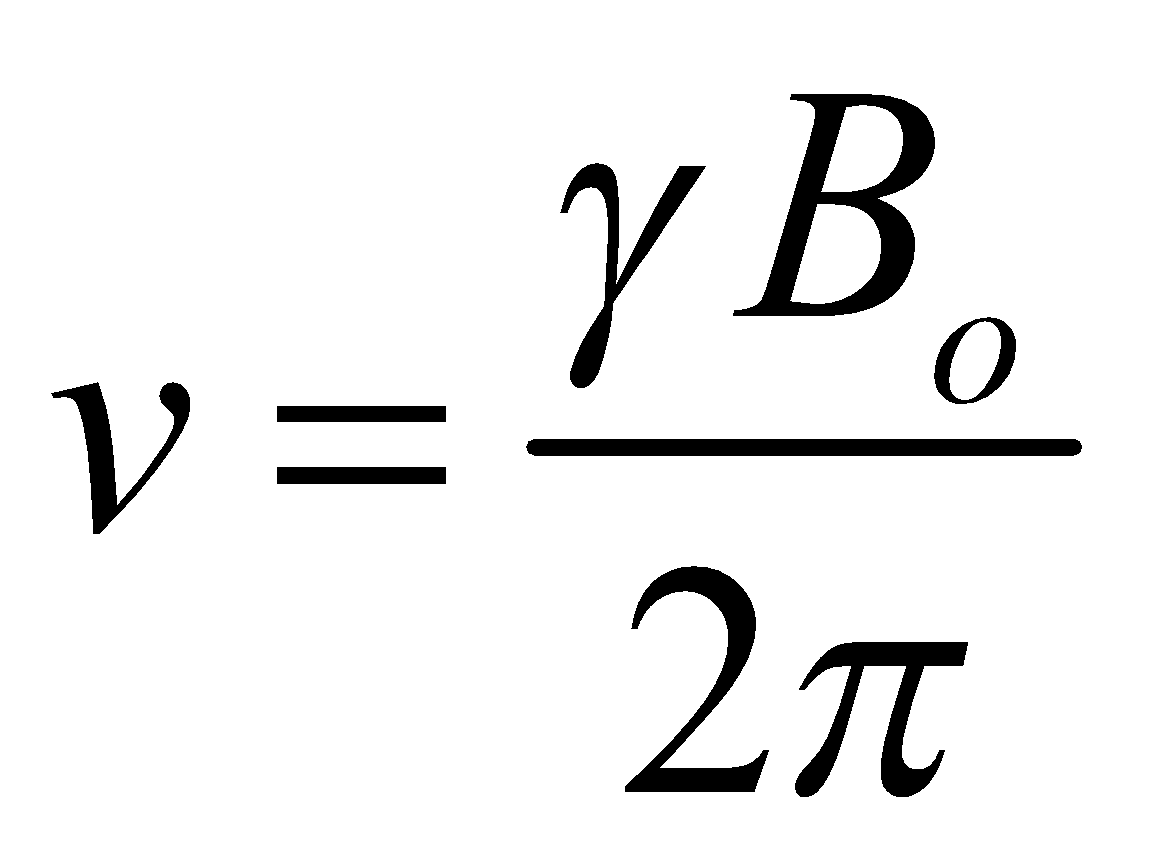
Where Bο is the external magnetic field experienced by proton and ϒ is the gyromagnetic ratio i.e.,
NMR instrumentation
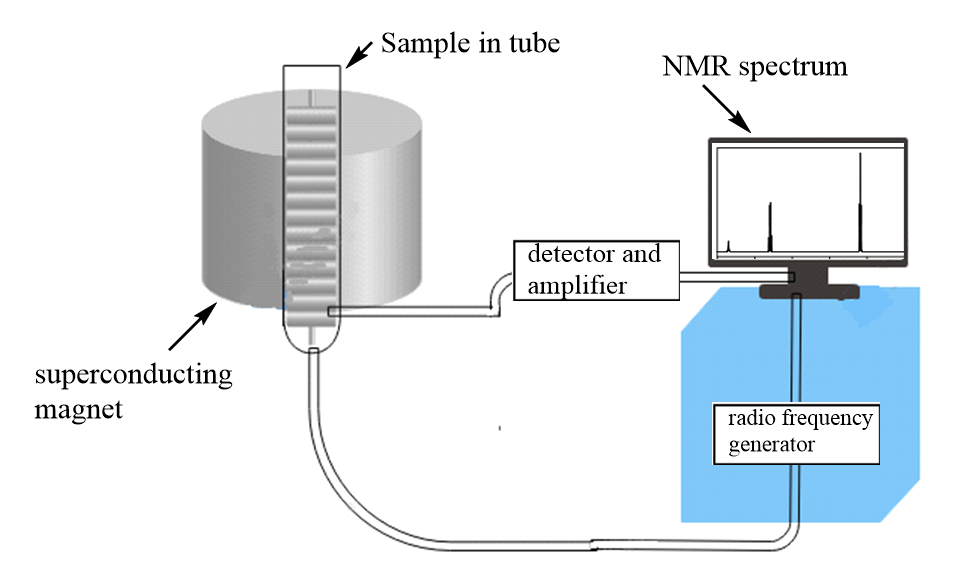
The instrumentation of NMR includes seven major parts which are listed as follows:
1. Magnetic coils: These coils are responsible to induce magnetic fields when current flows through them.
2. Sweep generator: It is used to produce the equal amount of magnetic field to pass through the sample.
3. Radio frequency transmitter: A radio transmitter coil which produces a short powerful pulse of radio waves.
4. Radio frequency receiver: It is used to detect radio frequencies emitted by nuclei when it reach back to its ground state.
5. Read-out systems: A computer system which analyses the data and records it.
The schematic diagram of the NMR instrumentation can be represented as follows:
NMR spectrum
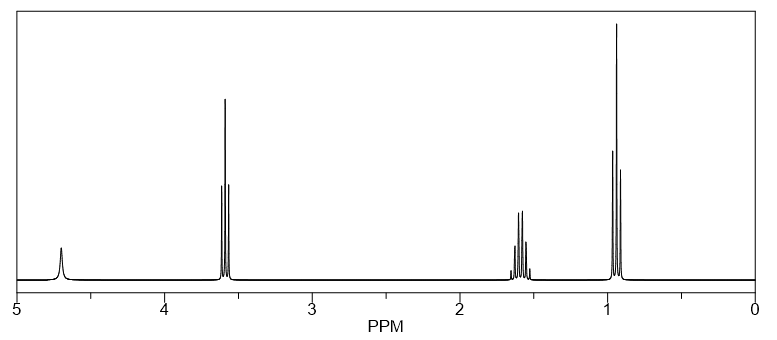
A computer system measures the change in intensity over time and converts it into intensity versus frequency data to produce a spectrum which is known as NMR spectrum or FT-NMR spectrum. It can be recorded in about 2 seconds and analysis can be done by using less than 5 mg of a compound. The NMR spectrum is drawn in reference to TMS i.e., tetramethylsilane because it has a strong, sharp resonance line with a chemical shift (we will discuss it later within this article) at low resonance frequency relative to almost all other 1H resonances. Thus, it does not interfere with other resonances and hence, is established as the internal reference compound for NMR spectrum. A NMR spectrum is usually represented as follows:
Solvents used in NMR:
The major solvents which are normally used for NMR spectroscopy are carbon tetrachloride (CCl4), carbon disulphide (CS2), deuterochloroform (CDCl3), Hexa deuterobenzene (C6D6) and deuterium oxide (D2O).
Chemical shift in NMR
A chemical shift is termed as difference in parts per million (ppm) between resonance frequency of an observed proton and TMS hydrogens. Mathematically, the chemical shift can be represented as follows:
Chemical shift, ∂=frequency of signal-frequency of reference/spectromete frequency × 106
In simple words, the chemical shift is a measure of how far the signal is from the reference compound i.e., TMS whose value is defined at zero position on ∂ scale.
Commonly Asked Questions
Shielding and deshielding of protons
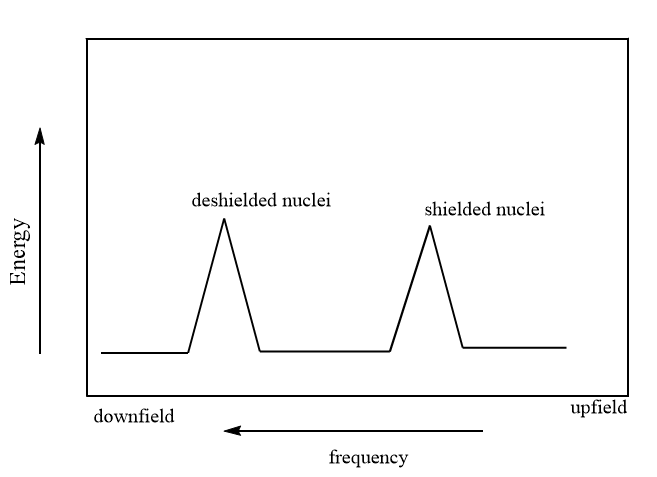
Generally, in NMR spectrum, the high electron density around a nucleus shields it from the external magnetic field and the signals are said to be upfield whereas the lower electron density around a nucleus Deshields the nucleus from external magnetic fields and the signals are said to be downfield. So, shielded protons come into resonance at lower frequencies than deshielded nuclei, as shown in the figure below:
Factors affecting chemical shift of NMR active nuclei
Electronegative groups: The electronegative groups attached to C-H the system decreases the electron density around the protons and deshielding occurs due to which the chemical shift of the nuclei increases. It can be explained with the help of an example as per following table:
Compound | Chemical shift |
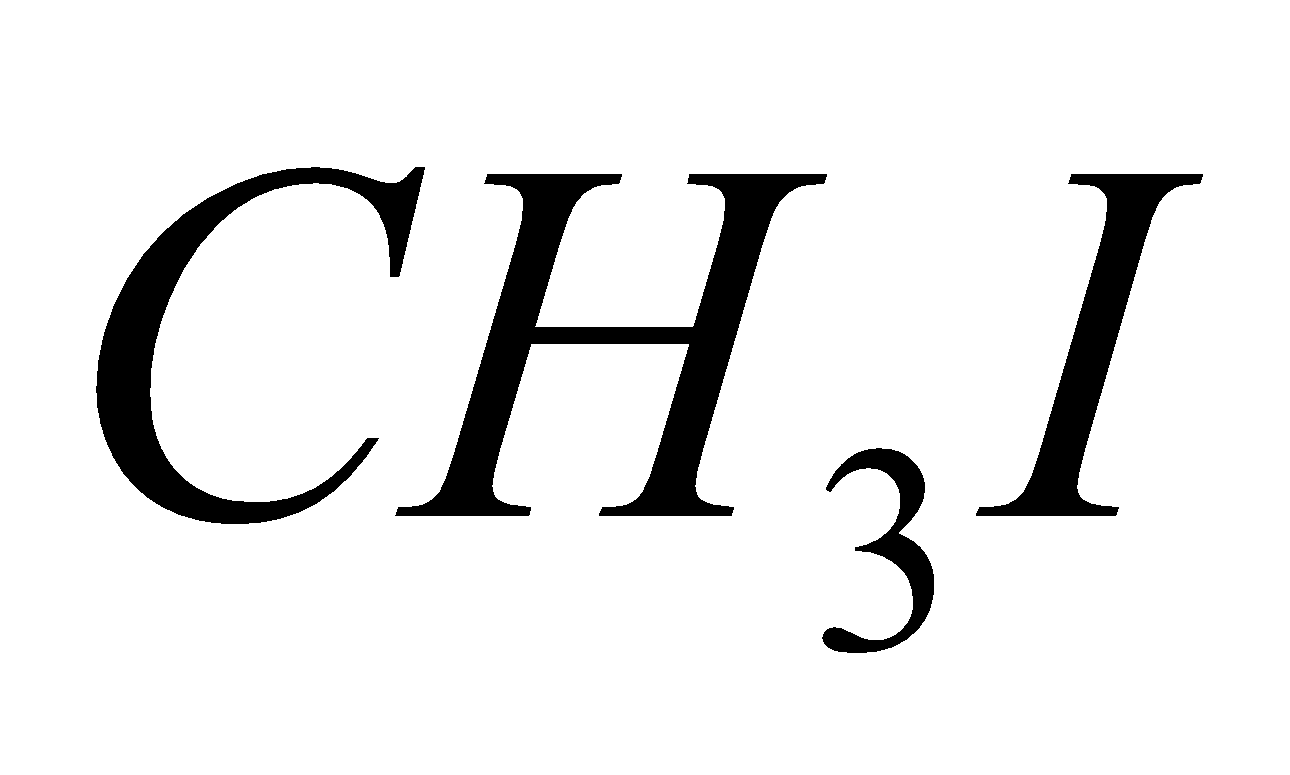 | 2.16 |
| 2.65 | |
| 3.10 | |
| 4.26 |
So, it is clear from the given values of chemical shift for methyl halides that as the electronegativity increases, the deshielding of the nuclei increases and chemical shift is observed at higher frequency regions.
Related Topics, |
Magnetic anisotropy of π- systems:
The word “anisotropic” here refers to “non-uniform”. So, basically magnetic anisotropy means that there exist a non-uniform magnetic field and electrons in π- systems like aromatic compounds, alkenes, etc. interact with the applied magnetic field which induces a magnetic field which causes anisotropy. It can cause both shielding and deshielding of protons depending upon the type of substituent groups attached with it. Example: Benzene.
Hydrogen bonding:
Protons which are involved in hydrogen bonding typically change the chemical shift values and greater the hydrogen bonding in the molecule, the more the proton will be deshielded and the higher will be the value of chemical shift.
Interpretation of proton 1H NMR spectroscopy
With the help of the proton NMR spectrum, we can indicate different kinds of protons present in a compound which are responsible for giving the number of signals in a compound. We can also indicate the position of signals i.e., about chemical shift and the electronic and magnetic environment of protons. Further it indicates the number of nearby nuclei, usually protons within a molecule using the n+1 rule which states that the number of protons in the nearby nuclei will be increased by 1. For example, the zero H atom as a neighbour will have a singlet as 1+0=1 and so on.
General rules for spin-spin coupling
1. Chemically equivalent protons do not have a tendency to show spin-spin coupling.
2. Only non-equivalent protons couple and are capable to show spin-spin coupling or splitting.
3. Protons on adjacent carbon will couple whereas the protons separated by four or more bonds will not couple.
Coupling constant
The coupling constant (J), which is independent of the operating frequency of the spectrometer, is defined as the distance between two adjacent peaks of a split NMR signal. Coupled protons tend to have the same coupling constant.
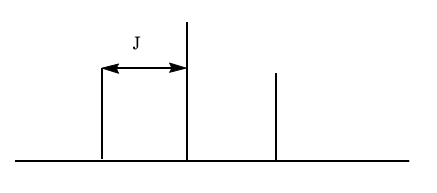
It is important to note that when two different sets of protons split a signal, the multiplicity of the signal is determined by using (Na+1)(Nb+1) for each set when the coupling constants for two sets are different. When the coupling constants are similar, then the N+1 rule is applied to both sets at the same time.
13C NMR spectroscopy
The number of signals in a 13C NMR spectrum corresponds to the number of different kinds of carbons in the compound. Carbons in an electron-rich environment produce low frequency signals whereas carbons close to electron withdrawing groups produce high frequency signals.
In 13C NMR spectrum, signals are not split by attached protons unless the spectrometer is run in a proton-coupled mode and it tells whether a signal is produced by CH3, CH2, CH or C.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Commonly Asked Questions
General applications of NMR spectroscopy
1. NMR is most commonly used in biology to study biofluids, cells, perfused organs and biomacromolecules such as nucleic acids (DNA, RNA, etc.), carbohydrates, proteins and peptides.
2. NMR is used in labelling studies in biochemistry.
3. NMR spectroscopy is widely used in food science.
Applications of proton (1H) NMR spectroscopy
NMR is majorly used for structure determination of compound in organic as well as inorganic compounds as follows:
Inorganic solids- Investigate the solid-state inorganic compounds like CaSO4.H2O
Organic solids: Investigate the properties like hydrogen bonding and ionization state for small organic compounds.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Commonly Asked Questions
Frequently Asked Questions (FAQs)