Periodic Trends in Ionic Radii in Modern Periodic Table - Atomic Radius with FAQs
What is a Periodic Table?
A periodic table is not anything, however the association of all of the recognised factors in a way in which the factors having comparable properties are categorised collectively in tabular form.
The improvement of the periodic desk passed off withinside the following series:-
Lavoisier Classification-
- What is a Periodic Table?
- Lavoisier Classification-
- 2. Prout’s Hypothesis-
- 3. Dobereiner Triad Rule-
- 4. Newland Octave Rule-
- 5. Lother Meyer's Curve-
- 6. Mendeleev's Periodic Table-
- 7. Modern Periodic Table -
- Atomic Radius –
- Ionic Radius trend
- Cationic Radius-
- Anionic Radius-
- Ionic size or Size of isoelectronic species:-
- Periodic trends in ionic radius-
- Periodic trends in ionic radius across the period:-
- Factors Affecting Atomic Size:-
- In a Period-
- In a Group-
- Lanthanide Contraction-
- S-Block variation-
The category turned into truly primarily based totally on factors being metals and non-metals.
Metals being the factors tending to lose electrons and non-metals being the factors tending to just accept electrons.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
2. Prout’s Hypothesis-
This speculation turned into primarily based totally on the presumption of all of the factors being made from hydrogen.
3. Dobereiner Triad Rule-
Three detail corporations have been made with the aid of using him having the equal chemical properties; in which the atomic weight of a center element is almost identical to the common weight of the first and third elements
4. Newland Octave Rule-
The factors have been organized in increasing order in their atomic loads and look at that the properties of each eighth detail have been just like the first detail.
5. Lother Meyer's Curve-
He plotted a curve among atomic extent and atomic weight of various factors and concluded that the physical properties of elements are periodic capabilities in their atomic weight.
6. Mendeleev's Periodic Table-
Mendeleev's periodic regulation states that the bodily and chemical houses of the factors are the periodic feature in their atomic weight. He turned into the primary scientist to set up the factors in a scientific way i.e. in horizontal and vertical columns.
7. Modern Periodic Table -
The Modern Periodic table turned into proposed with the aid of using Moseley primarily based totally on atomic number.
Moseley concluded that the physical and chemical houses of factors are periodic function in their atomic number. This manner that once the factors are organized in growing order in their atomic number factors having comparable properties receives repeated after normal intervals.
Atomic Radius –
The common distance of valence shell electrons from the nucleus is known as the atomic radius.
It may be very tough to degree atomic radius due to the fact the isolation of a single atom is tough. Also, there's no well-described boundary for the atom.
So, the atomic radius may be appropriately described as-
1. Half the internuclear distance (d) among atoms in a homoatomic molecule.
2. This internuclear distance is likewise known as bond length.
Based at the chemical bonds, the atomic radius is split into 4 categories:-
a. Covalent radius
b. Ionic radius
c. Metallic radius
d. Van der Waal’s radius
Let’s see what is ionic radius definition is-
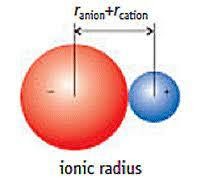
The radius of an atom's ion is called the ionic radius. Ionic radius is measured by dividing the lengths between the two nuclei of the respective ions based on their sizes. Hence, ionic radii are nothing but the distance from the nucleus of an ion up to which it has an influence on its electron cloud.
Ionic Radius trend
Ionic radius increases on moving top to bottom in periodic table.
Ionic radius is further classified into cationic and anionic radius. Table of ions and ionic radius table help us to know better examples of ions and its radius.
Cationic Radius-
A cation is a positively charged ion formed on losing electron(s) in a neutral state.
Cationic radius is always smaller than atomic radius because after losing electron(s), the number of electrons reduces, by the number of origins remains the same. This happens due to the increase in effective nuclear charge (Zeff). Hence, electrons are pulled towards the nucleus and the atomic radius decreases moreover after losing all the electrons from the outermost shell, the penultimate shell becomes the ultimate shell which is nearer to the nucleus so size decreases.
Size of cation ∝ 1/the magnitude of charge or Zeff
Eg : Fe > Fe+2 > Fe+3
Pb+2 > Pb+3
Anionic Radius-
Anionic radius is a negatively charged ion formed on gaining electron in the neutral state.
Anionic radius is always larger atomic radius because in an anion, the number of electrons are more than the umber of protons and this leads to increase in inter electronic repulsion further increasing the screening effect. So the effective nuclear charge reduces resulting in an increase in distance between electron and nucleus.
Eg: Flourine (Z = 9)
F | F- | |
Proton | 9 | 9 |
Electron | 9 | 10 |
Z/e | 9/9 = 1 | 9/10 = 0.9 |
As Zeff of fluorine anion (F-) is less than flourine atom (F)
Size of fluorine anion is greater than the parent atom i.e.
F- > F
Due to above mentioned reasons, cations are smaller and anions are larger in size than parent atom.
Atomic radius of chlorine is 175pm.
Read more :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Ionic size or Size of isoelectronic species:-
Chemical species containing same number of electrons but having different nuclear charge are called isoelectronic species. Atomic radius is inversely proportional to effective nuclear charge and hence, as the atomic radius increases, effective nuclear charge decreases for isoelectronic species.
Species | K+ | Ca+2 | S-2 | Cl- |
Z | 19 | 20 | 16 | 17 |
e | 18 | 18 | 18 | 18 |
Z/e | 19/18 | 20/18 | 16/18 | 17/18 |
Related Topics link, |
Periodic trends in ionic radius-
Periodic trends in ionic radius down the group:-
Ionic radius increases down the group in a periodic table. This happens because of the addition of extra shell ( number of electrons) by atoms.
Ions | Configuration | Ionic Radii (nm) |
Li+ | 2 | 0.076 |
Na+ | 2,8 | 0.102 |
K+ | 2,8,8 | 0.138 |
Ions | Configuration | Ionic Radii (nm) |
F- | 2,8 | 0.133 |
Cl- | 2,8,8 | 0.181 |
Br- | 2,8,18,8 | 0.196 |
Periodic trends in ionic radius across the period:-
Let us consider an example for better understanding of tis concept-
Let us consider 3rd period. We observe that atomic radius tends to increase first and then decreases gradually and then again increase and slowly decreases down. This happens because the initial elements have a tendency of forming cations and the elements present towards the end of the periodic table tend to form anions.
Period 3 | Na+ | Mg+ | Al+3 | P-3 | S-2 | Cl- |
No. of protons | 11 | 12 | 13 | 14 | 15 | 16 |
Electronic Configuration | 2,8 | 2,8 | 2,8 | 2,8,8 | 2,8,8 | 2,8,8 |
Ionic radius | 0.102 | 0.072 | 0.054 | 0.212 | 0.184 | 0.181 |
Factors Affecting Atomic Size:-
In a Period-
Atomic radius ∝ 1/Zeff ∝ Negative Charge/Positive
Charge
Li<Na<K<Rb<Cs
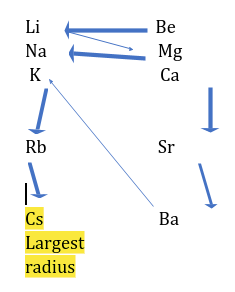
In a Group-
Atomic radius ∝ number of shells
Atomic size decreases from left to right in a period as Zeff increases.
LI > Be > B > C > N > O > F
Atomic size decreases from top to bottom in a group as number of shells are increased.
Li < Na < K < Rb < Cs
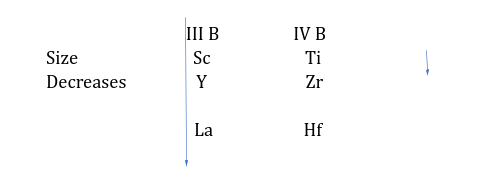
Lanthanide Contraction-
Outermost electronic configuration of inner transition elements is (n-2)f1-14,(n-1)s2p6d0-1,ns2 (n=6 or 7)
Electrons enters in (n-2)f orbitals.
Because of complicated structure of f orbital and due to poor shielding f electrons, the outermost shell electrons get attracted towards the nucleus.
In 1st,2nd,3rd, transition series, radii:- 3d>4d≈5d
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
S-Block variation-
Also check-
Frequently Asked Questions (FAQs)
A cation having lost its electron is smaller in size than Parent atom.
An anion having gained electron is larger in size than
parent atom.
The regular gradation in properties from top to
bottom and from left to right in a period is called
periodicity.
The ionic radius of magnesium ion (Mg2+) is 173 pm.
The ionic radius of Chlorine anion (Cl− ) is 175 pm.
Francium (Fr) is the element showing highest ionic radius having value 348 pm.
Also Read
02 Jul'25 06:01 PM
02 Jul'25 05:59 PM
02 Jul'25 05:59 PM
02 Jul'25 05:58 PM
02 Jul'25 05:58 PM
02 Jul'25 05:58 PM
02 Jul'25 05:53 PM
02 Jul'25 05:35 PM
02 Jul'25 04:44 PM