Polymers - Notes, Topics, Formula, Books, FAQs
Monomers, when combined to form large, long-chain molecules, form polymers. The word Polymer is made up of two words: poly and mer. The word poly means many, and mer means unit. Thus polymer is a bigger molecule made up of various smaller molecules, therefore, polymers are also known as macromolecules. Polymers are long chains that consist of repeated monomer units. We use polymers daily, from the tires of cars, DNA in blood, to the clothes we wear; polymers are everywhere. Polymers come in various forms, from stretchiness like rubber, flexibility like polythene, and toughness like metals and all these forms fulfill the various requirements of industries.
This Story also Contains
- Important Topics of Polymers
- Overview of The Chapter
- Classification of Polymers
- Types of Polymerization Reactions
- The Molecular Mass of Polymers
- Biodegradable Polymers
- Polymers of Commercial Importance
- How to prepare for Polymers?
- Important PYQS
- Prescribed Books
Important Topics of Polymers
Polymers - Types, Classification, Properties, and Uses
Polymers are macromolecules formed by the repeated linkage of smaller units called monomers. They can be classified based on origin (natural, synthetic), structure (linear, branched, cross-linked), and polymerization mechanism (addition, condensation). Natural polymers like cellulose and synthetic polymers like PVC have diverse applications. Polymers possess unique properties such as flexibility, durability, and resistance to water and chemicals. They are widely used in industries, including textiles, packaging, construction, and electronics.
Natural Rubber and Synthetic Rubber
Natural rubber is primarily composed of polyisoprene and is obtained from latex. Its elasticity and water resistance make it versatile, but vulcanization with sulfur is required to enhance its strength and durability. Synthetic rubber like neoprene and SBR (styrene-butadiene rubber), is made from petroleum-based monomers. Synthetic rubber exhibits improved resistance to heat, chemicals, and wear. Both Natural rubber and Synthetic rubber are important for manufacturing tires, footwear, and industrial goods.
Polyester
Polyesters are synthetic polymers formed through condensation polymerization of diols and dicarboxylic acids. The most common example is polyethylene terephthalate (PET). Polyesters are known for their durability, lightweight nature, and resistance to environmental conditions. Their properties make them suitable for applications in textiles, packaging materials (bottles), and insulation films.
Biodegradable Polymers
Biodegradable polymers are environmentally friendly materials that decompose under the action of microorganisms. Examples include polylactic acid (PLA) and polyhydroxybutyrate (PHB). These polymers are increasingly used in applications where environmental sustainability is crucial, such as agriculture, packaging, and medical devices. Their ability to minimize plastic waste has made them significant in addressing global pollution challenges.
Overview of The Chapter
In this chapter, you will study the various types of polymers that we use in daily life. In the following section, the classification of polymers is done using different methods.
Classification of Polymers
Polymers are classified on the basis of various categories as given below:
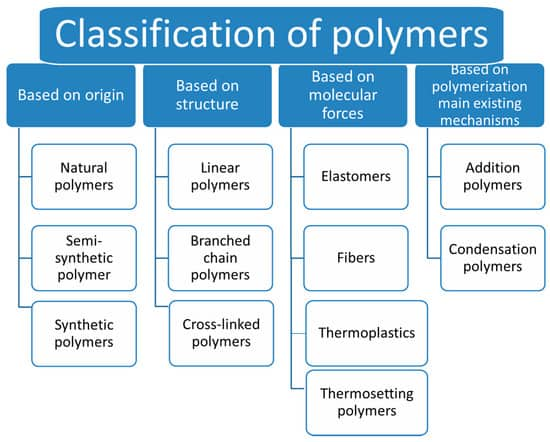
(i) Classification of Polymers on The Basis of Source: In this category, polymers are classified on the basis of source of origin:
-
Natural Polymers: These are the polymers that are directly obtained from nature. For example, protein, starch, rubber, etc.
-
Semi-synthetic Polymers: These are those polymers that are prepared in the laboratory by making some modifications in the natural polymers. For example, cellulose nitrate, cellulose acetate, etc.
-
Synthetic Polymers: These are completely man-made polymers and developed in the laboratory. Some common examples are plastic, nylon 6,6, etc.
(ii) Classification of Polymers on The Basis of The Structure of Polymers:
-
Linear Polymers: These are those polymers that have long and straight chains but no branches. Some common examples include high-density polythene, polyvinyl chloride, etc.
-
Branched-chain polymers: These are those polymers that have long chains but also have some branches. Some examples include low-density polythene.
-
Cross-linked or Network polymers: These are the polymers that have a long straight chain, but these chains are connected through strong covalent bonds. Examples are bakelite, melamine, etc.
(iii) Classification of Polymers on The Basis of Mode of Polymerization:
-
Addition polymers: These are those polymers that are formed by the repeated addition of monomers. The addition polymers are of two types, viz:
(i) Homopolymers: These are the polymers that are formed by the addition of the same monomers. For example:
$\mathrm{nCH}_2=\mathrm{CH}_2 \rightarrow\left(\mathrm{CH}_2-\mathrm{CH}_2\right)_{\mathrm{n}}$
(ii) Copolymers: The polymers formed by the addition of different monomers are known as copolymers. For example Buna-S, Buna-N, etc. -
Condensation polymers: These are those polymers that are formed by the repeated condensation reaction of two different bi-functional or tri-functional monomeric molecules. In these reactions, small molecules such as water, hydrogen chloride, etc eliminate. Some common examples include nylon 6,6, terylene, etc.
$\mathrm{nH}_2 \mathrm{~N}\left(\mathrm{CH}_2\right)_6 \mathrm{NH}_2+\mathrm{nHOOC}\left(\mathrm{CH}_2\right)_4 \mathrm{COOH} \rightarrow\left[\mathrm{NH}\left(\mathrm{CH}_2\right)_6 \mathrm{NHCO}\left(\mathrm{CH}_2\right)_4 \mathrm{CO}\right]_{\mathrm{n}}+\mathrm{nH}_2 \mathrm{O}$
(iv) Classification of Polymers on The Basis of Molecular Forces:
-
Elastomers: These are types of polymers in which the polymer chains are weakly bound to each other. Because of this weak interaction, these polymers have an elastic property. Some crosslinks are also present between the chains, due to which these polymers are able to regain their original position. Some examples include buna-S, buna-N, etc.
-
Fibers: These are thread-forming polymers. These polymers have high tensile strength and high modulus. They have strong intermolecular forces and thus have a crystalline nature. Some examples include polyamides, polyesters, etc.
-
Thermoplastic polymers: These polymers have a long chain of molecules with some branches. These molecules have the property to be softening on heating and hardening on cooling. Their intermolecular forces of attraction are intermediate between elastomers and fibres. Some common examples include polythene, polystyrene, etc.
-
Thermosetting polymers: These are highly branched chain polymers. These polymers cannot be reused. Some common examples include bakelite, urea-formaldehyde resins, etc.
Types of Polymerization Reactions
There are basically two types of polymerization reactions that you have to study in this chapter.
- Addition polymerization or chain-growth polymerization: In this type of polymerization reaction, the addition of the same or different type of monomers occurs. These monomers are unsaturated in nature and their addition leads to the long-chain polymers. Examples include high-density polyethylene, Teflon, etc.
- Condensation polymerization or step-growth polymerization: In this type of polymerization reaction, the repetitive condensation reaction of two bi-functional monomers takes place. This polymerization reaction results in the elimination of small molecules like water, alcohol, etc. Some examples include nylon 6,6, terylene, etc.
The Molecular Mass of Polymers
The molecular mass of polymers differs in different samples. The size and mass of any polymer depend on the number of monomers present in the reaction mixture. The properties of polymers depend upon their size and mass. Thus, the molecular mass of any polymer is always measured in terms of the average.
Biodegradable Polymers
Most of the polymers available to us are non-biodegradable. Thus, it has been a major environmental issue to degrade these non-biodegradable polymers. These polymers take a very long time to degrade and thus create many problems for the environment. To eliminate all these problems, some new biodegradable polymers have been developed as mentioned below:
- Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV)
- Nylon 2–Nylon 6
Polymers of Commercial Importance
Other than the polymers that have been described, there are further many more other polymers that commercial importance. These are described in the table below along with their uses.
| Name of Polymer | Monomer | Uses |
| Polypropene | Propene | Manufacture of ropes, toys |
| Polystyrene | Styrene | wrapping material, manufacture of toys |
| PVC | Vinyl chloride | Manufacture of raincoats, handbags, water pipes |
| Urea-formaldehyde resin | Urea and formaldehyde | Manufacture of unbreakable cups |
| Glyptal | Ethylene glycol and Phthalic acid | Manufacture of paints and lacquers |
| Bakelite | Phenol and formaldehyde | For making combs, electrical switches |
How to prepare for Polymers?
-
This chapter is part of organic chemistry. It is completely theory-based. You are not supposed to memorize any formulas and numerical practice for getting a good hold on this chapter.
-
In this chapter, you need to remember the names of some important polymers, and you also need to take care of the classification section
- This chapter is not very lengthy, it is easy, and remembering the diagrams of polymers is not necessary.
Important PYQS
Question: $\mathrm{nCH}_2=\mathrm{CH}_2 \rightarrow\left(\mathrm{CH}_2 \mathrm{CH}_2\right)_{\mathrm{n}}$
Polymer formed by above reaction is called-
1) Homopolymer
2) Copolymer
3) Elastomer
4) Cross-linked polymer
Answer:
As we have learned
Homopolymers -
A polymer formed by single monomeric species.
Hence, the answer is the option (1).
Question: In which of the following is polymer chain held by weak intermolecular forces?
1) Thermosetting polymer
2) Thermoplastics
3) Elastomers
4) Fibers
Answer:
As we have learned
An elastomer is a polymer chain that is held by weak intermolecular forces. Elastomers are rubber-like materials that are known for their high elasticity and ability to return to their original shape after being deformed
Hence, the answer is the option (3).
Practice more questions from the link given below
Prescribed Books
For this chapter, first, the NCERT book is best for initial level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you: Morrison and Boyd, and R.K. Gupta by Arihant Publication. Meanwhile, in the preparation, you must continuously give mock tests for the depth of knowledge. Our platform will help you to provide a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Also Read:
Frequently Asked Questions (FAQs)
Polymers are classified based on their origin, structure, and thermal behaviour. Natural polymers include proteins, cellulose, and synthetic polymers include nylon and polyethylene.
The degree of polymerization is the number of monomeric units in the polymer chain. The degree of polymerization affects the physical properties of polymers like strength, viscosity, and melting point. The degree of polymerization is directly proportional to molecular weight.
The word Polymer is made up of two words: poly and mer. The word poly means many, and mer means unit. Thus polymer is a bigger molecule made up of various smaller molecules; therefore, polymers are also known as macromolecules.
The common properties of polymers are durability, lightweight, flexibility, and resistance to chemicals. These properties vary based on their composition and structure. Some polymers are rigid while others are soft.
The molecular weight of a polymer can be calculated by using the formula:
- Molecular weight = Degree of polymerisation x Molecular weight of a molecule
Questions related to
On Question asked by student community
Correct Answer: Only 1,2 , and 4
Solution : The correcrt option is 4 i.e "Only 1, 2, and 4."
Explanation:
Let's refer to the following lines of the passage:
The device has a very simple structure consisting of specially designed nanocomposite polymers and contact electrodes and can generate a few Milliwatt (Mw) power, which is sufficient to power small electronic devices like watches, digital thermometers, radio frequency transmitters, healthcare sensors, pedometers.
The above statement clearly mention the watches, pedometers and radio frequency transmitters.