Thermosetting Polymers - Examples, Properties, Classification, FAQs
Polymer is said to be made up by the combination of two words called poly and mer where poly represents many and mer have the meaning parts which resemble that polymer is said to be like many parts this can be explained on the basis that a single polymer is made up of many repeating units of monomers and monomer is said to be a single part of any unit.
This Story also Contains
- Classification of Polymers:
- Properties of polymers:
- Thermosetting polymers examples
- Difference between thermosetting polymers and thermosetting plastics:
Monomer is also made up of two words called mono + mer where mono means single and mer means part this implies that monomer is known as the single part of any unit when these single units of monomer combine with each other and further give rise to a long chain polymers or three dimensional compounds and the whole process by which monomer will combine to give polymer is known as polymerization. Hence polymerization is defined as the process in which small monomers will joined with other through the process called addition polymerization.
Also read -
Classification of Polymers:
1. On the basis of their availability
Polymers are basically divided into three parts on the basis of its availability which can be defined as follows:
A. Natural polymers: These are those polymers which are available naturally or we can say which can be easily provided by plants and animals. The main examples of natural polymers are protein, cellulose, rubber etc.
B. Semi-synthetic polymers: These are those types of polymers which are occurred in nature but need some modifications to enhance their properties and uses so these will undergo chemical modification in laboratory by scientists. Cellulose nitrate or cellulose acetate is known as semi-synthetic polymers.
C. Synthetic polymers: These are also known by the other name called man made polymers as these types of polymers are prepared by man in laboratory. The main examples of synthetic polymers are nylon 6,6, polyethers etc.
2. On the basis of structure
Polymers can also be divided into three parts on the basis of their structure formation these can be discussed as follows:
A. Linear polymers: Those polymers in which monomers are joined in long straight chain are known as linear polymers like PVC i.e. poly vinyl chloride the main use of these type of polymers is to making long pipes and cables.
B. Branched chain polymers: In this case linear chain form some branches then this type of polymers are said to have branched chain like structures and termed as branched chain polymers. Low density polythene is one of the example of branched chain polymers.
C. Cross linked polymers: These are those polymers which are made up of bifunctional or trifunctional monomers and these contain a very strong covalent bond in them as co pare to other two types. The main examples of cross linked polymers are Bakelite and melamine.
Properties of polymers:
Physical properties:
1. The tensile strength of the polymer improves as the chain length and cross-linking increases.
2. Polymers do not melt; instead, they transition from crystalline to semi-crystalline form.
Chemical properties:
1. The polymer is enabled with hydrogen bonding and ionic bonding, resulting in improved cross-linking strength when compared to ordinary molecules with distinct side molecules.
2. The polymer's great flexibility is due to the dipole-dipole bonding side chains.
3. Van der Waals interactions between chains in polymers are known to be weak, yet they provide the polymer a low melting point.
Thermosetting polymers: Thermosetting meaning is defined as those polymers which are made up of cross-linked long chains of molecules. These are of rigid nature.
Formation of thermosetting plastics: This can be explained by the following diagram:
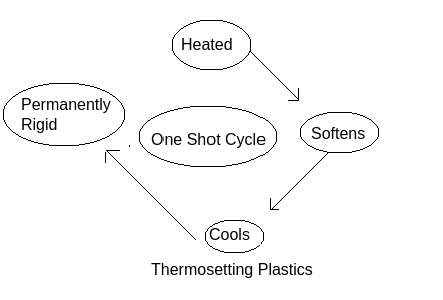
At first when thermosetting plastic gets heated it becomes soft in nature due to which it can be mould into any shape but when it get cold it will get fixed shape which can-not be regained easily where thermosetting meaning is to setting into a fixed shape with the help of heat and these are thermally stable polymers. Melamine is a thermosetting plastic.
Uses of thermosetting plastics: It can be used in electrical switches, kitchen wares, epoxides, vulcanized rubbers etc.
Thermosetting polymers examples
There are many examples of thermosetting polymers are Bakelite, vulcanized rubbers, epoxy resin, vinyl ester resin and polyurethane etc.
While talking about polymers in chemistry one thing generally confuse that thermosetting polymers or thermosetting plastics is these are similar to each or different one? These are seems to be similar but these are completely separate substances with distinct properties and applications. There are two main types of polymers known by the name thermosetting and thermoplastic polymers which can be distinguished primarily by their molecular bond and reaction towards heat.
When it comes to the distinctions between thermoplastic and thermosetting plastics, the fundamental difference between the two is that thermoplastic materials often have low melting points, allowing them to be readily remolded or recycled. On the other hand thermosetting plastic are said to have polar opposite. They can tolerate extreme temperatures and, once hardened, cannot be reformed or recycled, even when heated.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 15 Polymers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 15 Polymers
- NCERT notes Class 12 Chemistry Chapter 15 Polymers
Difference between thermosetting polymers and thermosetting plastics:
1. Formation: Thermosetting polymers can be synthesized by the process called addition polymerization while thermosetting plastics are made up by the process called condensation polymerization.
2. Procession: Injection molding, extrusion, blow molding, thermoforming, and rotational molding are all methods for processing thermoplastics. Compression molding and reaction injection molding are two methods for processing thermosetting plastic.
3. Bonding: Secondary bonds exist between molecular chains in thermoplastics. Primary bonds hold molecular chains together in thermosetting polymers, which are held together by strong cross-links.
4. Melting points: Thermoplastics generally have low melting point as well as low tensile strength on the other hand thermosetting plastics generally contain primary bonds which are in between molecular chains and these are attracted to each other through strong cross link polymers.
5. Molecular weight: Thermoplastics are of lower molecular weight while thermosetting having higher molecular weight.
Examples: Thermosetting polymers are Bakelite, vulcanized rubbers, epoxy resin, vinyl ester resin and polyurethane etc while thermosetting plastic examples are Teflon, Acrylic, Nylon etc.
There are many uses of polymers which can be discussed as:
1. Polymers are generally used in many industries like in textiles, plastics, aircrafts etc.
2. These are also used for making many household things like ropes etc. or toys.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: