Solutions - Topics, Books, FAQs
Have you ever wondered why salt disappears when mixed with water and how sugar dissolves in your tea? What really happens to the particles when a substance mixes completely with another? You will find these answers in this chapter, named solutions. Solutions are a part of our everyday life, from seawater and soft drinks to air and alloys.
This Story also Contains
- Important Topics of Solutions
- Overview of Chapter
- Previous Year Questions Of Solutions
- Some Preparation Tips of 'Solutions'
- Prescribed Books for Solutions

A solution is a homogeneous mixture of two or more substances whose composition can be varied within particular limits. The term homogenous mixture can be defined as a mixture where the composition and properties are steady throughout the mixture. Solution consists of solvent and solute, in which the solvent is the one present in a larger amount, whereas the solute is present in a smaller amount. There are various common examples of solutions that we see in our daily life, such as saltwater, which is formed when we mix salt in water. Vinegar is obtained when we mix acetic acid with water.
Important Topics of Solutions
Types of Solution
Solutions are homogeneous mixtures of two or more components. The Type of Solution is decided by the physical states of solute and solvent, and solutions are categorised as solid, liquid, or gaseous. For example, a sugar solution is a liquid solution, while air is a gaseous solution.
.png)
Expression of Concentration of Solution
The concentration of a solution indicates the amount of solute present in a given quantity of solvent or solution. Common expressions include molarity (moles of solute per litre of solution), molality (moles of solute per kilogram of solvent), mole fraction, and percentage composition.
Vapour Pressure of Solution
The vapour pressure of a solution is the pressure exerted by its vapour when in equilibrium with its liquid phase. It depends on the nature and quantity of the solute and solvent. Non-volatile solutes lower the vapour pressure of the solution compared to the pure solvent.
Formula - $P_{\text {solution }}<P_{\text {solvent }}^0$
Where,
$-P_{\text {solution }}$ = vapour pressure of the solution
- $P_{\text {solvent }}^0$- Psolvent 0= vapour pressure of pure solvent
Ideal Solution
An ideal solution follows Raoult’s Law, where the total vapour pressure is directly proportional to the mole fraction of components. Ideal Solutions exhibit no enthalpy change or volume change on mixing. Examples include benzene and toluene mixtures.
Raoult’s Law
Raoult’s Law states that the partial vapour pressure of a component in a solution is equal to the product of its mole fraction and its vapour pressure in the pure state. Raoult’s Law explains the colligative properties and is crucial in studying non-ideal solutions.
Formula:
$P_A=X_A P_A^0$
Where:
- $P_A$ = partial vapour pressure of component A
- $X_A$ = mole fraction of component A
- $P_A^0$ = vapour pressure of pure component A
For a two-component solution:
$P_{\text {total }}=P_A+P_B=X_A P_A^0+X_B P_B^0$
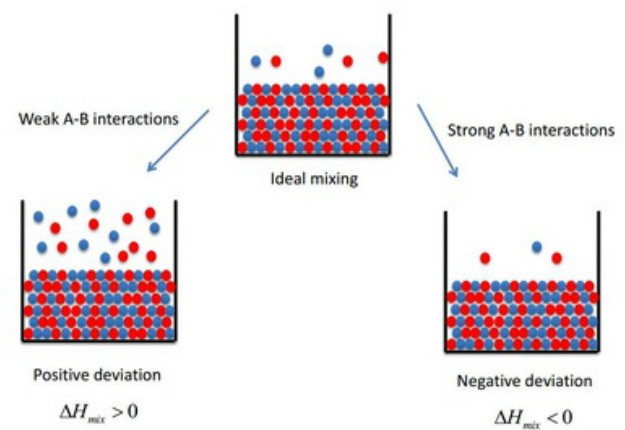
- Ideal and non-ideal solutions- Ideal solutions follow Raoult’s Law at all concentrations and there is no change in volume or enthalpy upon mixing. The interactions between A-A, B-B, and A-B are same whereas non-ideal solutions deviate from Raoult’s Law , either a positive deviation or a negative deviation, depending on A-B interactions, wheather they are weaker or stronger than A-A and B-B.
Positive deviation: $P_{\text {solution }}>X_A P_A^0+X_B P_B^0$
Negative deviation: $P_{\text {solution }}<X_A P_A^0+X_B P_B^0$
Azeotropic Mixture
Azeotropes are binary mixtures that boil at a constant temperature and behave as a single substance during distillation. An azeotropic mixture is formed due to strong deviations from Raoult's Law and can be either minimum or maximum boiling azeotropes.
Elevation in Boiling Point
Adding a non-volatile solute to a solvent increases its boiling point. Elevation in boiling point is a colligative property and occurs because the solute lowers the solvent’s vapour pressure, requiring more heat to reach the boiling point.
$\Delta T_b=K_b \cdot m$
Where:
$\Delta T_b=$ Elevation in boiling point
$K_b=$ Molal boiling point elevation constant of the solvent
$m=$ Molality of the solution
Depression in Freezing Point
The freezing point of a solution decreases when a solute is added. Depression in the freezing point is a colligative property which is used in applications like antifreeze solutions, where freezing is prevented by lowering the freezing point.
$\Delta T_f=K_f \cdot m$
Where:
$\Delta T_f=$ Depression in freezing point
$K_f=$ Molal freezing point depression constant of the solvent
$m=$ Molality of the solution
Osmotic Pressure
Osmotic pressure is the pressure required to stop osmosis, the movement of solvent molecules through a semipermeable membrane. It depends on the concentration of solute particles in the solution and is significant in biological processes.
$\Pi=M R T$
Where:
$\Pi=$ Osmotic pressure
$M=$ Molarity of the solution
$R=$ Universal gas constant
$T=$ Temperature in Kelvin
Reverse Osmosis
Reverse osmosis occurs when a pressure greater than the osmotic pressure is applied to a solution, forcing solvent molecules through a semipermeable membrane in the opposite direction. Reverse osmosis is widely used for water purification.
Isotonic, Hypertonic, and Hypotonic Solutions
Isotonic Solutions have equal osmotic pressure to a reference solution, such as body fluids. Hypertonic Solutions have higher osmotic pressure, causing water to move out of cells. Hypotonic Solutions have lower osmotic pressure, causing water to move into cells.
Van’t Hoff Factor and Abnormal Molar Mass
The Van’t Hoff factor accounts for the effect of ionization or association of solutes on colligative properties. It is used to calculate abnormal molar masses in cases where solutes dissociate (e.g., NaCl) or associate (e.g., acetic acid).
Solubility and Henry’s Law
Solubility refers to the maximum amount of solute that can dissolve in a solvent at a given temperature and pressure. Henry’s Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas. This principle is critical in industries like carbonated beverages and scuba diving.
Overview of Chapter
There are various kinds of binary solutions exist in nature as given in the table below:
|
Types of Solutions |
Solvent |
Solute |
Examples |
|
Gaseous |
Gas |
Gas |
A mixture of Oxygen and Nitrogen |
|
Gas |
Liquid |
Humidity | |
|
Gas |
Solid |
Camphor in Nitrogen gas | |
|
Liquid |
Liquid |
Gas |
Carbon Dioxide in Water |
|
Liquid |
Liquid |
Milk dissolved in water | |
|
Liquid |
Solid |
Sugar dissolved in water | |
|
Solid |
Solid |
Gas |
Hydrogen in Palladium |
|
Solid |
Liquid |
An amalgam of mercury with sodium | |
|
Solid |
Solid |
Brass (an alloy of copper and zinc) |
In this chapter, there are various important topics that you must understand completely:
(i) Solubility: The maximum ability of a substance to get completely dissolved in a solvent. When the solute is solid and the solvent is liquid, then only temperature affects the solubility. With the increase in temperature, the solubility increases. But pressure has no effect in this case as both solids and liquids are incompressible. But is the solute is gas, then pressure also is an important factor that plays a major role in insolubility. For this case, Henry's law is given which states that - "At constant temperature, the solubility of a gas is directly proportional to the partial pressure of a gas present above the solution".
(ii) Vapour pressure of liquid solutions: In this concept, you learn the vapour pressure of volatile liquids in solution when taken in a closed vessel. This phenomenon is explained by Raoult's law, which states that - "The vapour pressure of each volatile liquid present in the solution is directly proportional to the mole fraction of that liquid present in solution".
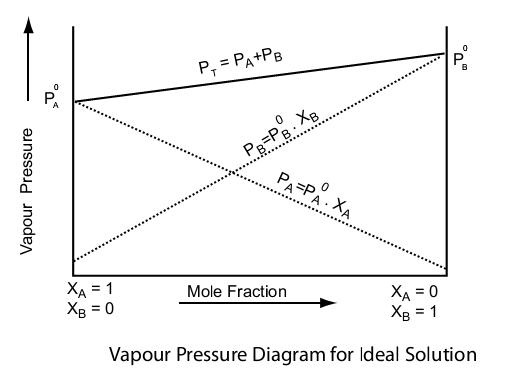
Mathematically, Raoult's law can be expressed as follows:
$p_{\text {total }}=p_1^0+\left(p_2^0+p_1^0\right) x_2$
where, $p_1^0$ = vapour pressure of pure component 1
$p_2^0$ = vapour pressure of pure component 2
x2 = mole fraction of component 2
(iii) Ideal and non-ideal solutions: Ideal solutions are those solutions which obey Raoult's law at all ranges of concentrations. Whereas the non-ideal solutions are those which do not obey Raoult's law at all ranges of concentrations. The vapour pressure of non-ideal solutions is always higher or lower than as predicted by Raoult's law and thus we say that the solution is exhibiting a positive or negative deviation, respectively.
(iv) Colligative properties: The properties of solutions which depend only on the number of solute particles present in the solution are known as colligative properties.
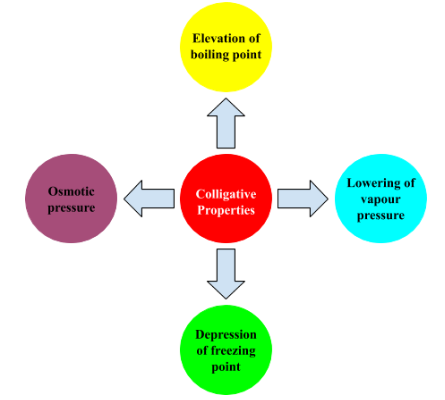
- Relative lowering of vapour pressure: The actual vapour pressure of a solvent in a solution is less than that of the pure solvent. Raoult explained this behaviour and established that this lowering of vapour pressure depends only on the concentration of the solute particles. Mathematically, it can be expressed as follows: $\Delta P_1 P_1^0=P_1^0-P_1 P_1^0=x_2$
- Elevation of the boiling point: The boiling point is the point of temperature when the vapour pressure of a substance is equal to the atmospheric pressure. This also depends upon the number of solute particles present in the solution.
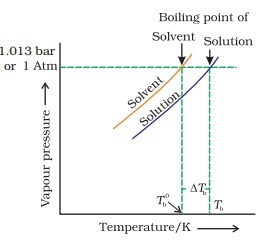
Mathematically, it can be expressed as follows: $\Delta \mathrm{T}_{\mathrm{b}}=\mathrm{K}_{\mathrm{b}} \mathrm{m}$
Kb = Boiling point elevation constant
- Depression of freezing point:
Freezing Point: It is the temperature at which the liquid and the solid form of the same substance are in equilibrium and have the same vapour pressure. A Solution freezes when its vapour pressure is equal to the V.P. of pure solid solvent. Due to the lower vapour pressure of the solution, the solid form of a solution separates out at a lower temperature.
$\Delta T_f=T_{f^{\circ}}-T_f$

- This is also termed cryoscopy and depression of freezing point (ΔTf)
- It can be measured by Beckmann's thermometer method and Rast's method.
- For a dilute solution, ΔTf is directly proportional to the molality (m) of the solution.
- Hence $\Delta T_f \propto m$
ΔTf = Kf m
If the molality of the solution is one, then
ΔTf = Kf
$\Delta \mathrm{T}_{\mathrm{f}}$ and M can be found out by using these relations.$\Delta T_f=K_f \frac{w}{M} \times \frac{1000}{W}$
$M=\frac{K_f \times w \times 1000}{\Delta T_f \times W}$
Here w= Weight of solute
W= Weight of solvent
$K_f$ = Molal depression constant or cryoscopic constant
M= Molar mass of Non-volatile solute.
$\mathrm{K}_{\mathrm{f}}=\frac{\mathrm{RT}^2}{1000 \mathrm{~L}_{\mathrm{f}} \text { or } \Delta \mathrm{H}_{\text {fusion }}}$
Here $\mathrm{L}_f$ or Δ$\Delta \mathrm{H}_{\mathrm{f}}$ = latent heat of fusion.
NOTE: The value of Kv or Kf depends only on the nature of the solvent and not on the nature of the solute.
Abnormal Mass: This concept says, that when a solute is dissolved in a liquid then the solute does not dissociate completely as expected instead it dimerizes and thus the molar mass of the solute becomes double. Such deviation of molar mass from the actual value is known as abnormal molar mass.
To deal with the case of an abnormal mass, a Vant Hoff factor was introduced in 1880, which mathematically is described as follows:
i= Normal Molar Mass Abnormal Molar Mass
Few more topics to read the concept based on solution chapter:
Previous Year Questions Of Solutions
Question 1: 20 mL of sodium iodide solution gave 4.74 g silver iodide when treated with excess of silver nitrate solution. The molarity of the sodium iodide solution is ________ M. (Nearest Integer value)
(Given : Na=23,I=127,Ag=108, N=14, O=16 g mol−1)
Ans. Let molarity of Nal solution be ×M
$\mathrm{NaI}+\mathrm{AgNO}_3 \rightarrow \mathrm{AgI}+\mathrm{NaNO}_3$
Moles of Agl formed$=\frac{4.74}{235}=0.02$
Moles of Nal$=\frac{20 \times \mathrm{x}}{1000}=0.02 \mathrm{x}$
$0.02 x=0.02$
x=1
∴ Molarity of Nal solution =1M
Hence, the answer is 1.
Question 2: The percentage dissociation of a salt (MX3) solution at given temperature (van't Hoff factor i= 2) is__________% (Nearest integer)
Ans. $\mathrm{MX}_3 \rightarrow \mathrm{M}^{+3}+3 \mathrm{X}^{\circ}$
$\begin{aligned} & \mathrm{i}=1+(\mathrm{n}-1) \alpha \\ & \mathrm{i}=1+(4-1) \alpha=2 \\ & \alpha=\frac{1}{3}=33.33 \% \approx 33 \%\end{aligned}$
Hence, the answer is 33%.
Question 3: Henry's law constant for $\mathrm{CO}_2$ in water is $1.67 \times 10^8 \mathrm{~Pa}$ at 298 K . Calculate the number of moles of $\mathrm{CO}_2$ in 540 g of soda water when packed under $3.34 \times 10^5 \mathrm{~Pa}$ at the same temperature.
Solution:
Given:
$k_H=1.67 \times 10^8 \mathrm{~Pa}$
$P=3.34 \times 10^5 \mathrm{~Pa}$
Mass of soda water $=540 \mathrm{~g}$
Density of soda water $\approx$ density of water $=1 \mathrm{~g} / \mathrm{mL}$
$\text { Volume }=\frac{540 \mathrm{~g}}{1 \mathrm{~g} / \mathrm{mL}}=540 \mathrm{~mL}=0.540 \mathrm{~L}$
Concentration of $\mathrm{CO}_2$ using Henry's law
$C=\frac{P}{k_H}=\frac{3.34 \times 10^5}{1.67 \times 10^8}=2.0 \times 10^{-3} \mathrm{~mol} / \mathrm{L}$
Moles of $\mathrm{CO}_2$ in 0.540 L
$n=C \times V=2.0 \times 10^{-3} \times 0.540=1.08 \times 10^{-3} \mathrm{~mol}$
Hence, the answer is 1.08x10-3 mol.
Question 4: A solution containing 15 g urea (molar mass $=60 \mathrm{~g} \mathrm{~mol}^{-1}$ ) per litre of solution in water has the same osmotic pressure (isotonic) as a solution of glucose (molar mass $=180 \mathrm{~g} \mathrm{~mol}^{-1}$ ) in water. Calculate the mass of glucose present in one litre of its solution.
Solution:
Osmotic pressure $(\pi)$
$\pi=C R T$
where $C$ is the molar concentration, $R$ is the gas constant, and $T$ is the temperature.
Given that:
mass of urea $=15 \mathrm{~g}$
- Molar mass of urea $=60 \mathrm{~g} / \mathrm{mol}$
- Volume of solution $=1 \mathrm{~L}$
Calculate the Molar Concentration of Urea:
Moles of urea ( $n_{\text {urea }}$ ) is calculated as:
$\begin{gathered}
n_{\text {urea }}=\frac{\text { mass of urea }}{\text { molar mass of urea }}=\frac{15 \mathrm{~g}}{60 \mathrm{~g} / \mathrm{mol}} \\
=0.25 \mathrm{~mol}
\end{gathered}$
Volume of the solution is 1 liter, then the molar concentration ( $C_{\text {urea }}$ ) is:
$C_{\text {urea }}=\frac{n_{\text {urea }}}{\text { volume }}=\frac{0.25 \mathrm{~mol}}{1 \mathrm{~L}}=0.25 \mathrm{~mol} / \mathrm{L}$
Since the solutions are isotonic, their osmotic pressures are equal:
$\pi_{\text {urea }}=\pi_{\text {glucose }}$
Using the formula for osmotic pressure:
$C_{\text {urea }} R T=C_{\text {glucose }} R T$
Since $R$ and $T$ are constants and the same for both solutions, they cancel out:
$C_{\text {urea }}=C_{\text {glucose }}$
Therefore:
$0.25 \mathrm{~mol} / \mathrm{L}=C_{\text {glucose }}$
Now for Mass of Glucose
we have molar mass of glucose $=180 \mathrm{~g} / \mathrm{mol}$
Molar concentration of glucose $\left(C_{\text {glucose }}\right)=0.25 \mathrm{~mol} / \mathrm{L}$
Moles of glucose in 1 liter of solution:
$\begin{aligned}
n_{\text {glucose }}=C_{\text {glucose }} & \times \text { volume }=0.25 \mathrm{~mol} / \mathrm{L} \times 1 \mathrm{~L} \\
& =0.25 \mathrm{~mol}
\end{aligned}$
Mass of glucose ( $m_{\text {glucose }}$ ) is:
$\begin{aligned}
m_{\text {glucose }} & =n_{\text {glucose }} \times \text { molar mass of glucose } \\
& =0.25 \mathrm{~mol} \times 180 \mathrm{~g} / \mathrm{mol}=45 \mathrm{~g}
\end{aligned}$
Therefore, the mass of glucose present in one liter of its solution is $\mathbf{4 5}$ grams.
Hence, the answer is 45g
Practice more questions from the link given below
For more questions to practice, the following MCQs will help in the preparation for competitive examinations
Some Preparation Tips of 'Solutions'
- This chapter is a part of the physical chemistry and it is very important from the numerical point of view. The theory part is less but you simply need to work with the formulas and questions of this chapter.
- There are some common terms used in this chapter like vapour pressure and mole fraction, so before coming to this chapter, first, you must finish the two basic chapters - "Mole concept" and "Gaseous state".
- You must have completely memorized all the formulas and must have a very good grasp of solving the numerical problems.
- Usually, most of the students like this chapter as it is very easy and straightforward. This chapter holds a good weight ok marks in board exams as well as in competitive exams like JEE and NEET.
Prescribed Books for Solutions
For this chapter, first, you need to finish the important topics of solutions class 12 thoroughly from the class 12th NCERT book and then simultaneously solve the examples and questions given in the book. Apart from this, if you want to prepare for the advanced level of competitive exams like JEE and NEET, you must prepare from the books - O.P. Tandon and R.C Mukherjee. Meanwhile, in preparation, you must continuously write mock tests to increase your depth of knowledge. Our platform will help you to provide a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Also check:
Frequently Asked Questions (FAQs)
- Molarity (M) is moles of solute per liter of solution. Its value changes with temperature because the volume of the solution changes.
- Molality (m) is moles of solute per kilogram of solvent. Its value is independent of temperature because mass does not change with temperature. Molality is generally preferred for expressing concentrations when dealing with colligative properties because colligative properties depend only on the number of solute particles, not on the volume of the solution, and they are often measured over a range of temperatures. Using molality ensures the concentration value remains constant regardless of temperature fluctuations.
Concentration expresses the amount of solute present in a given amount of solution or solvent. Common ways include:
- Mass Percentage (% w/w): (Mass of solute / Mass of solution) × 100
- Volume Percentage (% v/v): (Volume of solute / Volume of solution) × 100 (for liquid-liquid solutions)
- Mass by Volume Percentage (% w/v): (Mass of solute / Volume of solution) × 100
- Parts per Million (ppm): (Mass or volume of solute / Mass or volume of solution) × 10^6 (for very dilute solutions)
- Mole Fraction (χ): Moles of a component / Total moles of all components. (Sum of mole fractions is always 1).
- Molarity (M): Moles of solute / Volume of solution (in Litres). (Temperature dependent)
- Molality (m): Moles of solute / Mass of solvent (in kg). (Temperature independent)
In chemistry, a solution is a homogeneous mixture composed of two or more substances. "Homogeneous" means that the substances are uniformly distributed throughout the mixture, leading to a consistent appearance and properties throughout. You cannot distinguish the individual components by sight, even under a microscope.
A solution is typically composed of:
- Solute: The substance that is dissolved. It is usually present in a smaller amount.
- Solvent: The substance that dissolves the solute. It is typically present in a larger amount. Example: In saltwater, salt is the solute and water is the solvent.
Raoult's Law states that the vapor pressure of a solvent in a solution is directly proportional to the mole fraction of the solvent in the solution. Mathematically, it can be expressed as: Psolution =Xsolvent Psolvent where Psolution is the vapor pressure of the solution, Xsolvent is the mole fraction of the solvent, and Psolvent is the vapor pressure of the pure solvent. This law is applicable for ideal solutions and helps predict how the addition of solutes affects the vapor pressure of solvents.
Molarity is a measure of concentration that expresses the number of moles of solute per liter of solution. It is significant because it allows chemists to quantify the concentration of solutions, facilitating calculations and comparisons in chemical reactions and processes.
Solubility typically increases with temperature for most solids, meaning more solute can dissolve in a solvent at higher temperatures. However, the solubility of gases decreases with an increase in temperature. Understanding these variations is crucial for applications in fields such as pharmaceuticals and environmental science, where temperature management is essential for desired solubility levels.
The Van 't Hoff factor (i) is a measure of the number of particles a solute produces in solution. It is used to calculate colligative properties, such as boiling point elevation and freezing point depression. For non-electrolytes, i is typically 1, while for electrolytes, it equals the number of ions produced upon dissociation.
Colligative properties have several practical applications in everyday life. For example:
- Freezing point depression is utilized in making ice cream, as adding salt to ice lowers the freezing point, allowing for colder temperatures and faster freezing.
- Boiling point elevation is important in cooking, as adding salt to water increases the boiling point, allowing for higher cooking temperatures.
- Osmotic pressure is crucial in biological systems, such as maintaining the proper balance of fluids in cells and tissues. Understanding these properties enables chemists and engineers to design better processes in food preparation, pharmaceuticals, and various industrial applications.
An azeotropic mixture is a mixture of two or more liquids that has a constant boiling point and composition throughout the distillation process. This means that the vapor produced during boiling has the same composition as the liquid mixture, making it challenging to separate components by simple distillation. Azeotropes are significant in various industrial applications where precise separation of components is required.
Questions related to
On Question asked by student community
Hello,
You can get the Question Paper from this link : PU BA LLB Previous Year Question Papers
Hope it helps !
Hello,
Here is the link where you will get TN Quarterly Question Paper :
Tamil Nadu Class 11th Quarterly Exam Maths Question Paper 2025-26
Hope it helps !
Hello,
You can get Karnataka SSLC question papers from this link : Karnataka Mid-term SSLC Maths Question Paper 2025-26
Hope it helps !
Hello,
You can get NMMS Questions from these links :
Hope it helps !
For GATE ECE preparation, using previous years’ question papers is one of the best strategies because the exam tends to repeat concepts and problem patterns. You can find official GATE papers with solutions on the IIT organizing institute’s website as well as on NPTEL and other academic portal.
Check this link below: https://engineering.careers360.com/articles/gate-question-papers
Thank you!