Titration - Definition and their Types, Example, FAQs
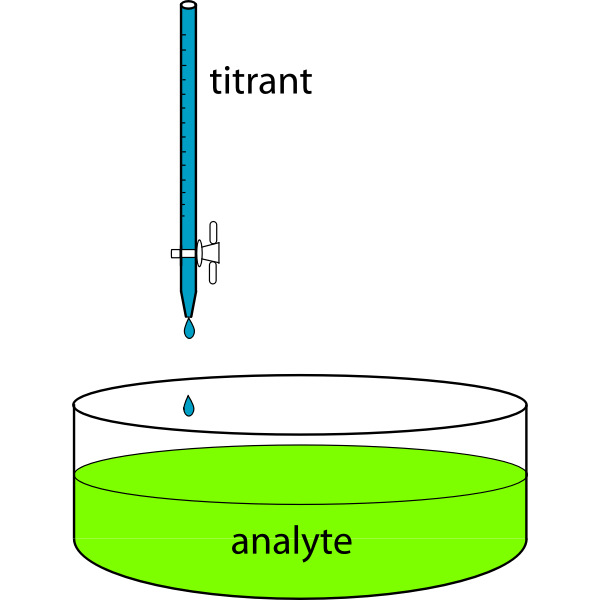
Have you ever wondered how chemists determine the exact concentration of an unknown solution? What process makes it possible to measure acidic or basic concentrations with accuracy? The answer is titration. Titration is a technique that is used to determine concentration. It is the technique that is used in analytical chemistry where the determination of concentration of unknown solution can be done with the help of known solution. Solution having known concentration are termed as titrant, on the other hand unknown concentration are termed as analyte.
This Story also Contains
- Titration
- Titration in Chemistry
- Types of Titration
- Acid-Base Titrations
- Complexometric titrations
- Types of Complexometric Titrations
- Applications Of Complexometric Titration
- Redox Titration
- Precipitation Titration
- What is Standardization in Chemistry?
- Some Solved Examples
Titration can be defined as adding the definite known proportion of another sample in the given unknown sample of different constituents to react with each other in a definite amount. This process can be conducted by gradually adding the other standard solution; such a type of chemical reaction is called titration. With the help of this we can usually find out the concentration of other unknown constituents.
Titration
Titration can be defined as the slow process of addition of one solution to any known concentration that is called titrator or titrant to a specified volume of another unknown solution concentration which is termed as titrand or analyte. The reaction continues until it reaches to neutralize the reaction, which can be seen by change in colour. The reaction can be analysed until some equivalence point is not reached.
The standard solution of titration: This is the known concentration solution, which can be added to other unknown solution during titration
The analyte: This is the unknown concentration of the solution, which can be added in order to achieve the end or equivalence point during titration.
The equivalence point: This is the point where the reactants complete the reaction and no further change can be observed after reaching this point in titration. It can be shown with the help of moles of standard solution to the moles of unknown concentration.
The end point: It can be indicated by some form of indicator, when it is added to the equivalence point.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Titration in Chemistry
It can be defined as a technique where the determination of unknown solutions can be done with the help of known concentration solutions. The main aim of the titration is to determine the equivalence point. The titrant is added with the help of burette.
Also read :
Types of Titration
It is generally used in quantitative and volumetric analysis. The volumetric analysis can be done for estimation of different types of titration. The different types of titration can be classified as follows:
-
Acid-base titration
-
Complexometric titration
-
Redox titration
-
Precipitation titration
With the help of above-mentioned types of titration we can easily identify the unknown concentration of the solution quantitatively as well as volumetrically.
Acid-Base Titrations
This titration depends on the neutralization reactions that occur in between acid and base. Here the acid strength can be identified using standard solution, the process called acidimetry. The strength of the base can be found with the help of standard solution of an acid, which is called alkalimetry. The method is quantitative analysis which is used in examining the acid base concentration by neutralising them in a standard solution more precisely with acid or base with known concentration. With the help of pH indicator we can easily monitor the development of acid-base reaction.
Example: Acid+ Alkali salt=Water
The titration occurs between an acid or a base and analyte. In such acid-base titration, a known reagent is mixed in the sample, so that it further reaches the required pH solution. The pH indicator here used is not for the neutral solution but for equivalent points. The value of pH depends on the strength of acid or base either equal to seven or greater or lesser than seven. The end point of the acid-base titration may suffer from hydrolysis to some extent. The acids or bases are weak in nature, which affects the concentration of hydrogen ions in the solution.
The two generally used indicators in such titrations are Phenolphthalein and methyl orange.
Complexometric titrations
At an equivalence point the undissociated complexes are being formed. No error can be detected in this type of titrations like as in precipitation titrations. Complexes are formed with the help of metal ions and ligands. Here the metal ions are Lewis acids whereas ligands are Lewis bases. Ligands are defined as electron-pair donors and they bond with metal ions to form coordinate covalent compounds.
Ligands can be classified as follows:
-
Unidentate: Only a single side ligand is present to form a complex.
Example: Cyanide ion
-
Bidentate: In this, two side ligands are present.
Example: Glycine and oxalic acid
-
Multidentate: In this, two or three side ligands are present.
Example: EDTA
Related Topics Link,
Types of Complexometric Titrations
Direct titration
-
Back titration
-
Displacement or substitution titration
-
Indirect titration
-
Alkalimetric titration
-
Miscellaneous titration
Applications Of Complexometric Titration
-
It can be used to determine the concentration of metal ions in a solution.
-
Used to determine the calcium present in food products.
-
Used to determine the concentration of bad metals in the environment.
Redox Titration
This titration is also known as oxidation-reduction reaction. In aqueous solution the electrons are transferred in the reaction. The titration are so named after the reagents, is as follows:
-
Permanent titrations
-
Dichromate titrations
-
Iodimetric or Iodometric titrations
Precipitation Titration
When the two substances are reacted to and brought into contact, so that insoluble precipitation forms, this titration is called precipitation titration. Examples of such reactions are when Cl is determined with the help of silver nitrate. Titrimetric analysis: This is the classical method where a solution of known concentration with suitable reagent when treated with suitable amount of reagents. This can help us in determining the substance in the solution. For the determination of the substance, the reagents are added till the equivalence amount of substance is not reached. Such analysis can be done with the help of two methods as follows:
-
Karl-Fischer titrator
-
Auto titrator
For titrimetric analysis it is to be kept in mind that the amount of substance which we have to determine react rapidly and completely with other reagents and should be fastly in stoichiometric proportion at the equivalence point. Acidimetry and Alkalimetry: The analysis through titrimetric can be carried out in the various types of reaction and it is basically seen in the neutralisation reaction. In the neutralisation reaction there is involvement of acids and bases. Here the solutions are standard in which acids(acidimetry) and bases(alkalimetry) are being used in these reactions. Concentration of the solution in titrimetric analysis can be quantitatively done with the help of molarity. Molarity is the number of moles that are dissolved in one litre of solution.
What is Standardization in Chemistry?
In titrimetric analysis the determination can be possible with the help of known concentration of the volume of the solution. This known concentration is accurate, and is called the standard solution. The standard solution will not react with the solvent in which it is dissolved as well as be stable at room temperature, it can directly be used in preparing the solutions.
Also check-
Some Solved Examples
Question 1 What is the primary purpose of using a phenolphthalein indicator in an acid-base titration?
A) To detect the concentration of the acid
B) To detect the concentration of the base
C) To mark the equivalence point by changing color
D) To neutralize the solution
Solution:
Phenolphthalein is commonly used in acid-base titrations because it undergoes a color change (from colorless to pink) in the pH range of 8.3 to 10.0, which corresponds to the equivalence point of many strong acid-weak base titrations. It marks the endpoint by changing color, indicating the completion of the titration.
Hence the correct answer is option (3)
Question 2: In a titration experiment, a 25 mL sample of hydrochloric acid (HCl) is titrated with a 0.1 M sodium hydroxide (NaOH) solution. If 30 mL of NaOH solution is required to reach the endpoint, what is the concentration of the HCl solution?
A) 0.08 M
B) 0.12 M
C) 0.15 M
D) 0.20 M
Solution:
Using the equation for neutralization:
$
\text { Molarity of acid }\left(M_1\right) \times \text { Volume of acid }\left(V_1\right)=\text { Molarity of base }\left(M_2\right) \times \text { Volume of base }\left(V_2\right)
$
Given:
- M₂ = 0.1 M (NaOH concentration)
- V₂ = 30 mL = 0.030 L
- V₁ = 25 mL = 0.025 L
Now, solve for $\mathrm{M}_1$ :
$
\begin{gathered}
M_1 \times 0.025=0.1 \times 0.030 \\
M_1=\frac{0.1 \times 0.030}{0.025}=0.12 \mathrm{M}
\end{gathered}
$
Hence the correct answer is option (2)
Question 3: Which of the following best describes the equivalence point in a titration?
A) The point where the amount of acid equals the amount of base.
B) The point where the amount of acid exceeds the amount of base.
C) The point where the amount of base exceeds the amount of acid.
D) The point where the color change of the indicator occurs.
Solution:
The equivalence point in a titration is the point at which the amount of acid completely reacts with the amount of base. It is reached when the stoichiometrically equivalent amounts of both reactants have been mixed.
Hence, the correction answer is option (1)
Frequently Asked Questions (FAQs)
The permanganometry and dichrometry are the two qualitative techniques that are used in qualitatively analysing in chemistry.The permanganates and dichromates are used in estimating the amount of unknown samples.
The major difference between acidimetry and alkalimetry is, in acidimetry measurement of strength of acids whereas in case of alkalimetry measurement of strength of bases occur.
The four basic parts are as follows:
Burette
White tile
pH indicator
Pipette
To the concentration of acid base by neutralising the solution with an acid or a base with the help known concentration.
Titrations are used to identify the unknown concentration of acid or base.