Air Composition Properties - Chemical Composition of Air, FAQs
Air is the invisible gaseous substance that surrounds the earth, it is mainly a mixture of oxygen and nitrogen. In this article, we will discuss what is the composition of air, the properties of air/characteristics of air, the composition of gases in atmosphere, and gases in air or air content.
This Story also Contains
- Introduction To Air Composition
- What Is The Composition Of Air
- Properties Of Air
- Importance of Air Composition
- Air Properties Table/Properties of Air Worksheet
- Properties Of Air Experiments
We know that matter occupies space. Air is also a matter and it is essential for our lives. Our planet Earth is covered by a blanket-like layer called the Atmosphere. Among all the other fundamental elements present in nature, it is vital because life cannot exist without air. So now let’s discuss the composition of air.
Introduction To Air Composition
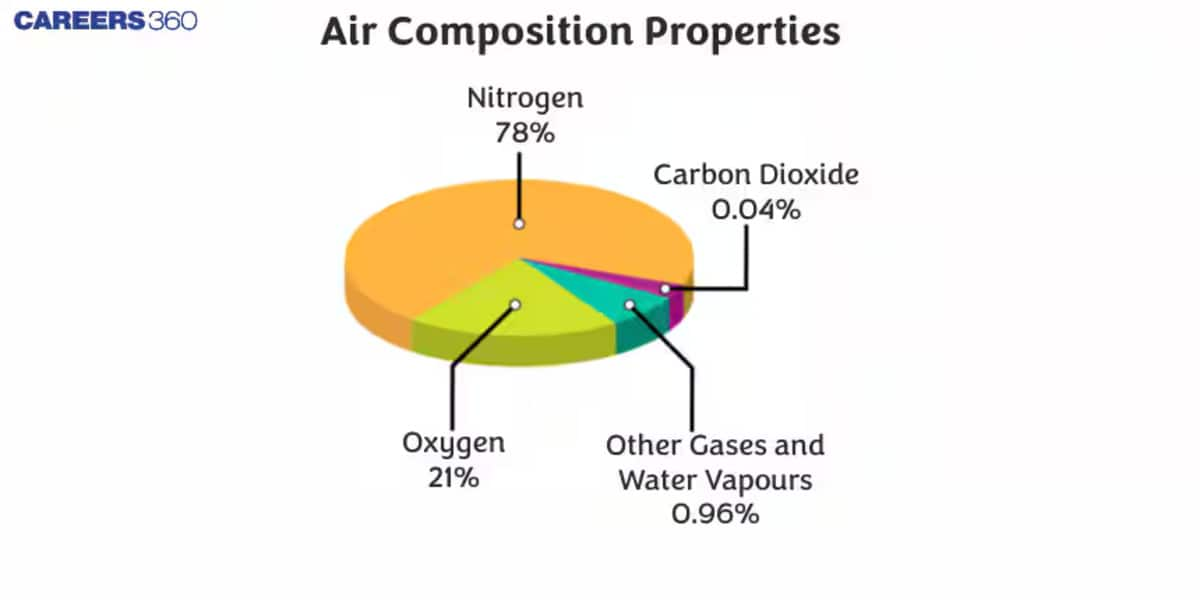
Composition of air by mass: The atmospheric air composition of the earth consists of a mixture of gases called the air. The gases are usually odorless and colorless. As a result, we cannot touch or see the air. We can only feel the air. The earth’s atmosphere consists of 78% of Nitrogen, 21% of oxygen, 0.93% of Argon, 0.04% of Carbon dioxide, and traces of helium, methane, neon, krypton, and hydrogen, as well as water vapor.
The composition of air remains the same up to a height of approximately 10,000 m. The composition of air diagram is given below:
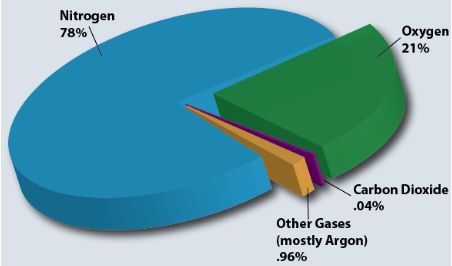
Other minor components of air
Some other compositions of air percentage are as follows:-
Sulphur dioxide(1.0ppm), Methane(2.0ppm), Nitrous oxide(0.5ppm), Ozone(0 to 0.07ppm), Nitrogen dioxide(0.02ppm), Iodine(0.01ppm), Carbon monoxide(0 to trace ppm), and Ammonia(0 to trace ppm).
What Is The Composition Of Air
Let us describe the main components of air, one by one:
Here are the main gases in the air.
- Nitrogen (N2)
Nitrogen is the major component that is found in air. It is the most abundant gas present on the earth. It is required for the growth of plants and animals. Nitrogen in air has a composition of 78%.
- Oxygen (O2)
The composition of Oxygen in air is 21% (approximately). Oxygen is required by all living organisms because they derive energy from food with the help of oxygen.
- Carbon dioxide (CO2)
Carbon dioxide occupies a small content of air around us. It is essential for plants and animals. Plants utilize carbon dioxide to make food during photosynthesis. The process of photosynthesis and respiration helps in maintaining the balance of oxygen and carbon dioxide in the atmosphere.
- Minor components in air percentage:
- Neon: 0.0018%
- Helium: 0.0005%
- Methane: 0.0002%
- Krypton: 0.0001%
- Hydrogen: 0.00001%
- Nitrous Oxide: 0.00003%
Properties Of Air
(i) Physical Properties
- Air occupies space.
- Air has mass.
- Heat affects the air.
- Air exerts pressure.
- Air is highly compressible.
- Altitude affects air.
- Air is colorless and odorless
- Air has low viscosity
- Air has several single boiling points
- Air is a good insulator
(ii) Chemical Properties
- Oxygen which is a main component of air is highly reactive.
- At high temperatures, nitrogen forms reactive compounds.
- Oxygen is a strong oxidizing agent
- Air reacts with metals to form oxides.
- Oxygen in air and moisture leads to corrosion of metals
- Oxygen contributes to the combustion process.
Importance of Air Composition
- Oxygen: It is vital for the respiration of living organisms. Oxygen aids to ozone layer formation.
- Nitrogen: Helps in plant growth through nitrogen fixation. Nitrogen in air also helps in protein synthesis.
- Carbon dioxide: It is an integral component of photosynthesis. It is also called a greenhouse gas for maintaining the earth's atmospheric temperature.
- Argon: Used in welding and insulation
The composition of air is important for supporting life and the ecosystem on Earth. It also helps in maintaining Earth's climate and temperature.
Now, as we discuss further the properties of air, a question arises Air shows the properties of both oxygen and nitrogen. Why? The answer to this question is air shows the properties of both oxygen and nitrogen because air is a mixture of gases. That’s why it will show the properties of both oxygen and nitrogen present in it.
Q. Which is not a property of air among the following options? (a)Air has mass. (b) Air occupies space. (c) Air is compressible. (d) Air is a compound.
Ans. (d) Air is a compound.
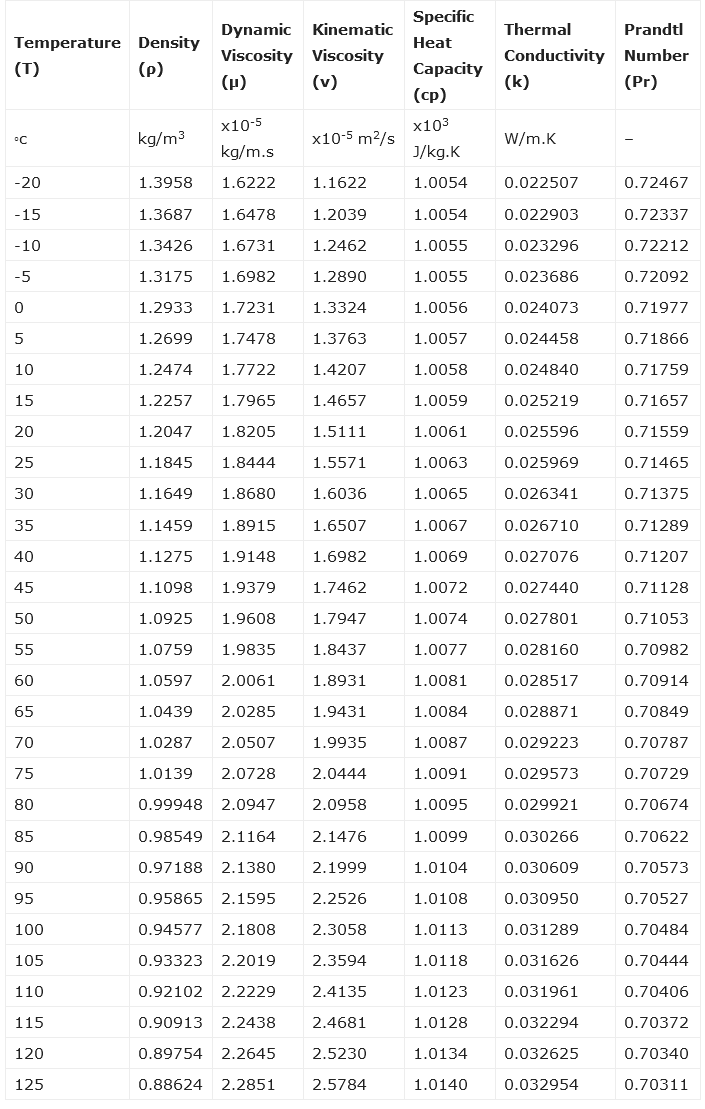
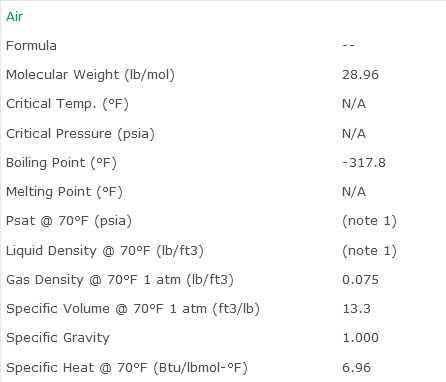
Air Properties Table/Properties of Air Worksheet
Properties Of Air Experiments
- Let’s take an empty glass bottle. Now, put the open mouth of the bottle into a container filled with water. It is observed that water does not enter the bottle when it is in an inverted position, as there is no space for air to escape. This shows that air occupies space.
- Let’s hang two balloons at the end of a thin straw. If we one balloon is burst then the straw will move towards the balloon that is left. This shows that air has mass.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subject
Gases in Air
Composition of gases in air: Nitrogen (N2 ) is the most abundant occurring natural gas, and it makes up about 78% of air. The second most abundant gas is oxygen and it is about 21%. Argon (Ar), the inert gas is the third most abundant gas at 0.93%. Some other gases like carbon dioxide (CO2), nitrous oxide (NO), neon (Ne), krypton (Kr), helium (He), methane (CH4), hydrogen (H2), xenon (Xe), ozone (O3), iodine (I2), carbon monoxide (CO), and ammonia (NH3), etc. are also present in the atmosphere.
Does air occupy space?
Or
How will you prove that air occupies space?
Let’s take an empty glass bottle. Now, put the open mouth of the bottle into a container filled with water. It is observed that water does not enter the bottle when it in an inverted position, as there is no space for air to escape. This shows that air occupies space.
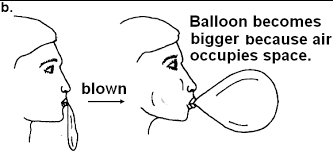
Air occupies space examples
Let’s take a balloon and fill it up. The air from ours lungs enters the balloons and it expands because air inside it is taking up the space.
Also read :
NCERT Solutions for Class 6 Science Chapter 15 - Air Around Us
Q. Why we can’t see air?
We can’t see air because it sends very little colour to our eyes. We see objects because they absorb some light wavelengths, and reflect others back to us. Objects appear to be the colour they reflect to our eyes.
We know that air is a mixture of gases and every gas has different wavelengths. When the light bump a molecule, it absorbs some colour and then reflect it back. The reflected light enters our eye and then we are able to see the object. But in case of air, wavelengths of light may pass by the molecules without hitting them.
When they do bump a molecule, it absorbs some colour and scatters some, spreading it out in all directions. Too little light reaches our eyes for us to notice. That’s why we can’t see air.
Physical Properties for Air
Frequently Asked Questions (FAQs)
(i) Air occupies space.
(ii)Air has mass.
Definition of composition of air: Earth’s atmosphere consists of a mixture of gases called the air. The gases are usually odourless and colourless. As a result, we cannot touch or see the air. We can only feel the air. The earth’s atmosphere consists of 78% of Nitrogen, 21% of oxygen, 0.93% of Argon, 0.04% of Carbon dioxide, and traces of helium, methane, neon, krypton and hydrogen, as well as water vapour. This is also known as Air composition of Earth.
Air occupies space.
Air has mass.
Heat affect the air.
Air exerts pressure.
Air is compressible.
Altitude affects air.
Troposphere
Water
Temperature
Pressure
Moisture
Wind
Yes, air is a matter. Hence it has mass.
Air content (ac) = Va / Vv
Where, Va= volume of air present in the pores
Vv =total voids volume in the soil mass