Charging Of Battery And Discharging Of Battery
Batteries are vital in our daily lives, powering devices like smartphones, laptops, and electric cars. The charging process involves taking energy from an external source, like a wall socket, and storing it as chemical energy within the battery. When you use your device, the discharging process occurs, converting that stored chemical energy back into electrical energy to power the device. Understanding how batteries charge and discharge is essential not just for keeping your devices running efficiently but also for extending their lifespan, ensuring that they are ready when you need them most. This is why practices like not overcharging your phone or properly maintaining an electric car’s battery are so important in everyday life.
This Story also Contains
- Emf of a Cell When the Cell is Charging and Discharging
- Equation of Cell
- Solved Examples Based on Charging of Battery And Discharging of Battery
- Summary
Emf of a Cell When the Cell is Charging and Discharging
Before moving to the charging and discharging, will study open and closed circuits.
What is an Open Circuit?

No current is taken from the cell.
The potential difference between
The potential difference between
What is a Short Circuit?

Two terminals of a cell are joined together by a thick conducting wire then,
Maximum current -
Equation of Cell
1. When supplying the current:
2. When the cell is being charged:
Recommended Topic Video
Solved Examples Based on Charging of Battery And Discharging of Battery
Example 1: The length of a wire of a potentiometer is 100 cm and the e.m.f. of its standard cell is E volt. It is employed to measure the e.m.f. of a battery whose internal resistance is 0.5 $\Omega$ If the balance point is obtained at $l=30$ cm from the positive end, the e.m.f. of the battery is :
1)
2)
3)
4)
Solution:
Using the principle of potentiometer
i.e
Hence, the answer is option (4).
Example 2: In the given circuit value of current (in A) through the circuit when the switch is open is

1) 0
2) 2
3) 1
4) 4
Solution:
Current through the circuit
i=0
wherein
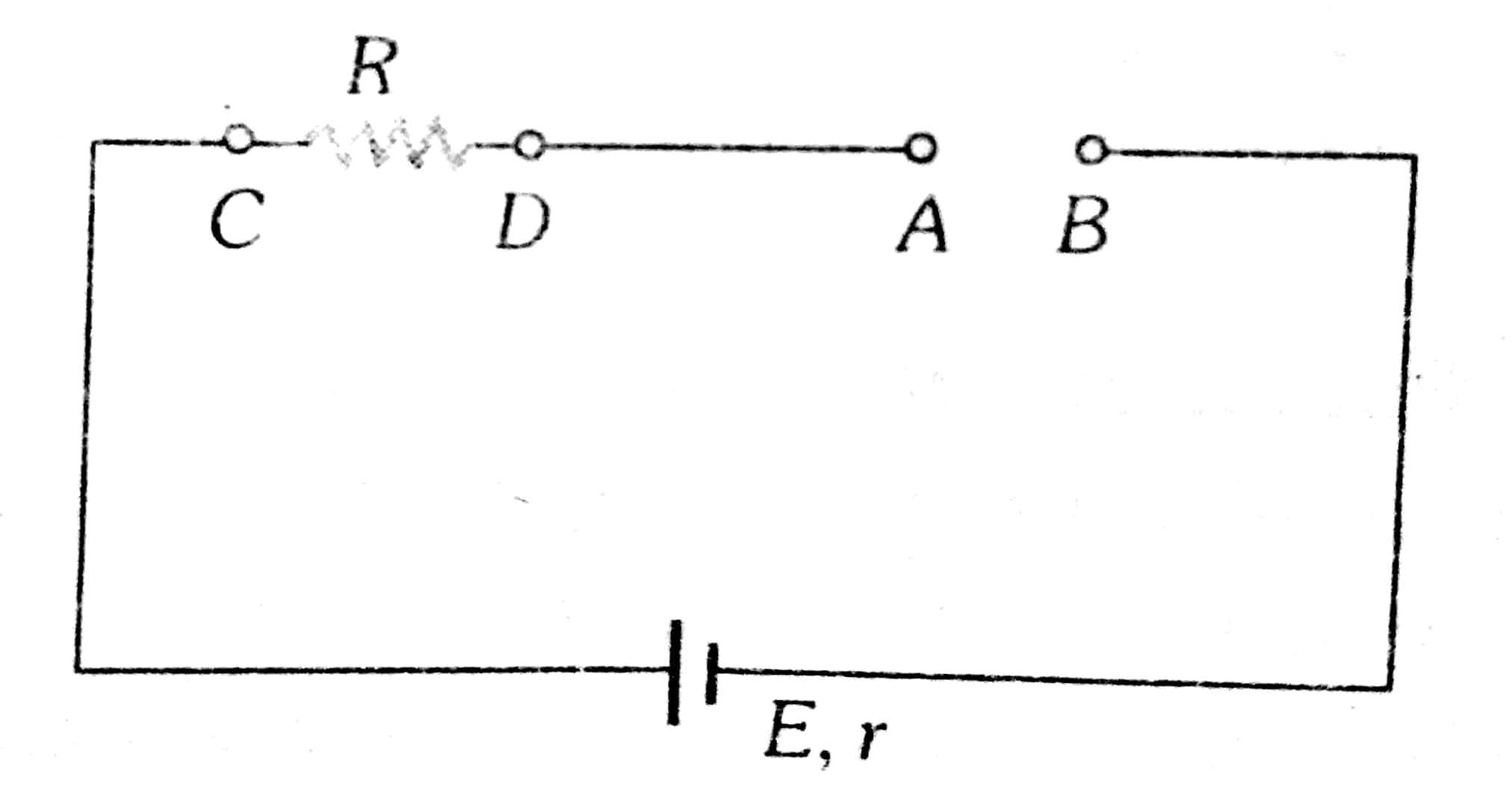
No current will pass through the resistor
Example 3: The potential difference between points A and B in the given circuit is

1)
2)
3)
4)
Solution:
As we learntPotential difference between
wherein
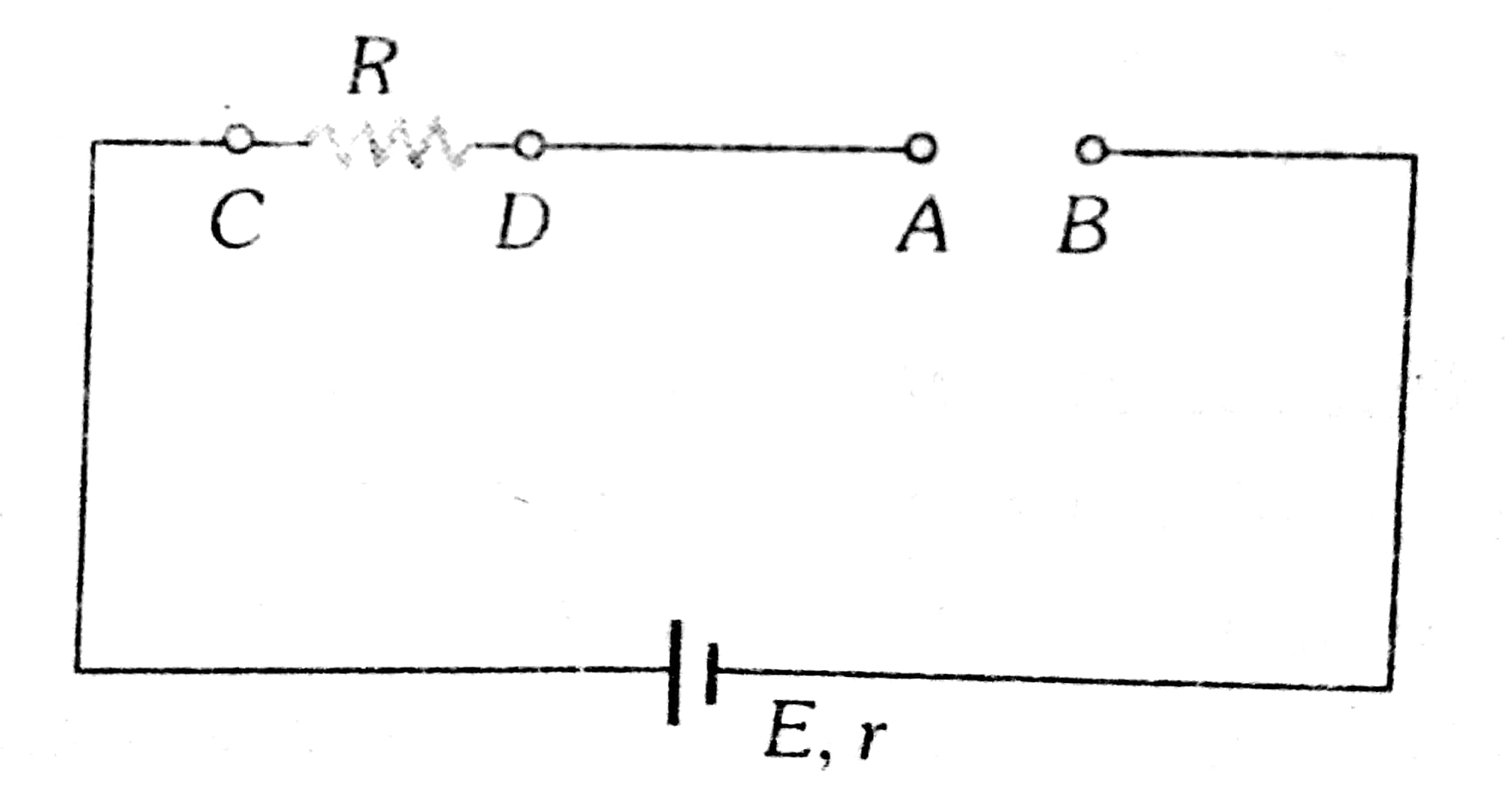
P.D. across the resistor is zero.
Hence, the answer is option (1).
Example 4: In the given figure current (in A) passing through

1) 0
2) 2
3) 2.5
4) 5
Solution:
Short circuit -Two terminals of a cell are joined together by a thick conducting wire
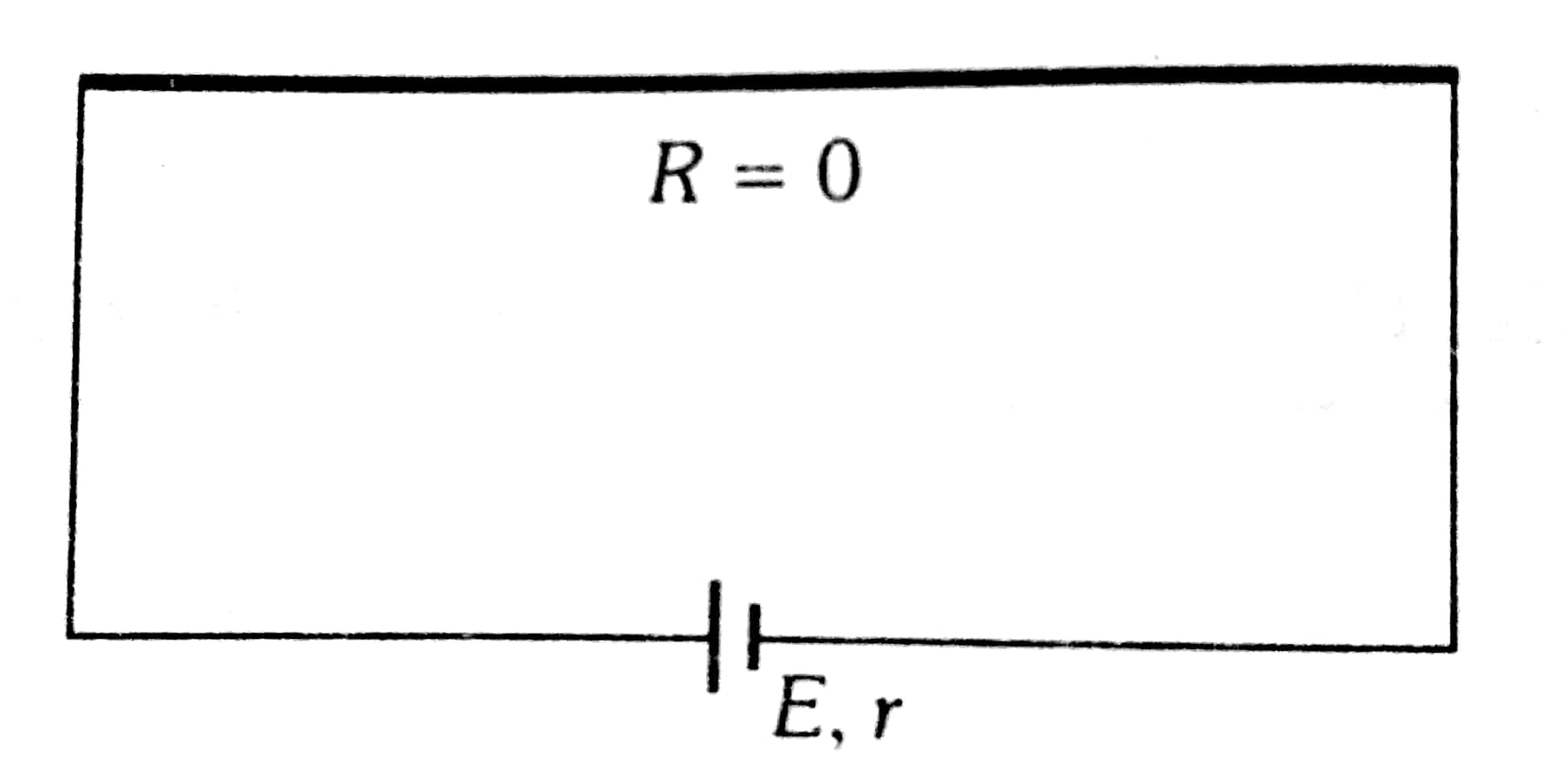
Since
So,
Example 5: If the terminal of a battery is short-circuited then the current flowing through it is:
1) zero
2) minimum
3) maximum
4) none of the above
Solution:
When a battery is short-circuited, the terminal voltage is zero. The current passing through the cell is the maximum.

Hence, the answer is option (3).
Summary
Supplying electrical energy to a battery for it to store energy for later use is called charging. The battery receives the input of electricity causing an electrical current to flow through it hence energy is stored in its cells through some chemical reactions. Discharging a battery occurs when one is using it to power a device or an appliance. The device is powered by electrical energy which is changed from the stored chemical energy.
Frequently Asked Questions (FAQs)
Decreased runtime, increased charging times, and reduced capacity.
Charging involves applying a voltage higher than the battery's voltage to drive current into the battery, causing chemical reactions that store energy.
Battery discharging occurs when the battery provides electrical energy to power a device or appliance.
Yes