Relation Between Bar and ATM
Bar and atm are very important in physics where students need to be familiar with pressure and the respective unit topics using both bar and atm of pressure. Both are pressure-measuring standard units and are commonly used in thermodynamics, fluid mechanics, and gas laws. One bar is slightly less than one atm. Learning to convert bars into atm or vice versa is indeed helpful in accurately solving all the problems.
What is Pressure?
Pressure is defined as the force applied per unit area on the surface of any object.
Force applied on an object refers to effects on the entire surface. This applies not only as a whole but also to a particular area of the object's surface. Effects of force are typically described in terms of pressure - that is, the amount of force acting on a unit area of surface. Hence, it indicates how much force is applied to a unit area. The same force then applied on a smaller surface causes greater pressure as compared to that exerted on a larger area.
Mathematically the pressure is stated as
$$
P=\frac{F}{A}
$$
Where,
$\mathrm{P}=$ Pressure
$\mathrm{F}=$ Force applied
A = Area on which pressure is applied
Types of Pressure
Atmospheric Pressure - Atmospheric pressure is the pressure which is applied by the weight of the atmosphere above us. It depends on the height above the surface.
Absolute Pressure - Absolute pressure is the pressure measured when it is measured relative to a vacuum or a situation of no pressure at all.
Differential Pressure - It is the difference between the pressure measured between two objects.
Gauge Pressure - It is defined as the pressure obtained by subtracting the atmospheric pressure from the pressure measured by the barometer.
Also, read
- NCERT Notes For All Subjects
- NCERT Exemplar Solutions for All Subjects
- NCERT Solutions for All Subjects
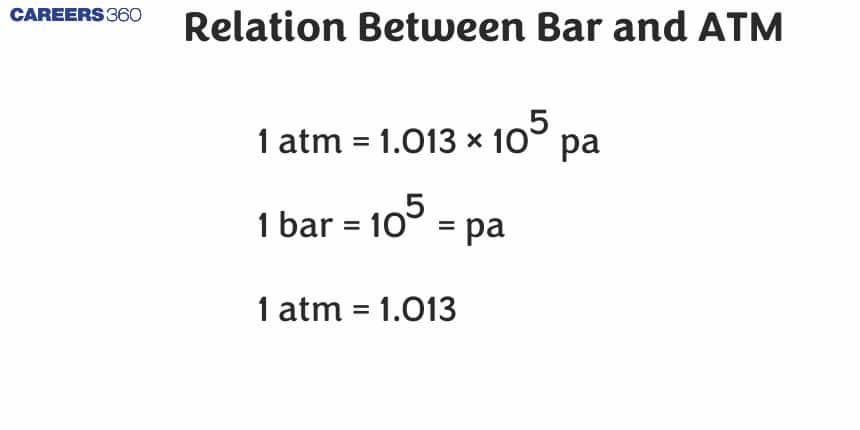
BAR to ATM Modification
The word bar is derived from the Greek word osος, baros, meaning weight. The official symbol of the unit is a bar which does not mean that the previous symbol given by the letter b has now declined and contradicts the use of b which indicates the unit bar but we can also say that it still faces it mainly as mb instead or rather there is a proper mbar to define millibar. Air or in other words atmospheric pressure is usually given in millibar. Where we can say that the normal atmospheric pressure is defined as 1013.25 mbar 101.325 kPa and then we see that the 1.01325 bar weighs 14.7 pounds as a square inch.
|
Related Topics |
Bar to Atm conversion
Conversion from Bar to atm helps solve problems. See the table given below for some of the most commonly used pressure values in the Bar scale and its equal value in the atm scale.
Conversion of BAR to ATM
1 Bar =0.986923 atm
ATM to BAR conversion
1 atm =1.01325 bar
Symbol Formula unit Basic
1 atm Pressure found in column air at sea. atm
$\begin{aligned} & 1 \mathrm{~atm}=101.325 \mathrm{kPa} \mathrm{atm} \\ & 1 \mathrm{bar}=100,000 \mathrm{~N} / \mathrm{m}^2\end{aligned}$
Frequently Asked Questions (FAQs)
A drying unit called a metric unit of pressure but not part of the SI International System of Units. We can say that it is defined to be exactly equal to 100,000 Pa per 100 kPa or we can say that it is slightly smaller than the pressure of the current atmospheric pressure on planet Earth at sea at approximately 1.013 bar.
It is generally said to correspond to the pressure exerted by a vertical column of mercury such as a 760 mm barometer which is also 29,9213 inches high. One of the most common cases is called the single state equal to 101,325 pascals or we can say that the new energy per square meter is about 14.7 kilograms per inch.
A pascal equal to Pa is a standard unit of pressure. That would mean that the normal atmospheric pressure is known as 1 atm of pressure and is equal to 760 mmHg and 101.3 kPa. The pressure that is atmospheric pressure is also often referred to as pounds or not to a square inch called psi. The so-called atmospheric pressure is 14.7 psi.
As a general guideline we have learned we can say that almost all sea level pressures live between 950 milligrams and 1050 milligrams with high sea level pressure i.e. reading drops between 980 milligrams and 1040 milligrams.
Both bar and torr are pressure units. 1 torr = 1 mm. Of Hg col. :. 1 bar = 760 torr.
It has long been known that air has a weight. The atmosphere weighs 14.7 pounds by six inches [6 cm] of ocean power. This means that an air column 1 inch long as air, can weigh 14.7 pounds. We usually call this 1 Atmosphere of pressure, or 1 ATM.
Also Read
24 Dec'24 03:48 PM
02 Dec'24 12:39 AM
20 Nov'24 03:00 PM
16 Nov'24 12:51 AM
14 Nov'24 05:37 PM
12 Nov'24 01:14 PM
12 Nov'24 09:24 AM
25 Sep'24 05:44 PM
25 Sep'24 05:44 PM
25 Sep'24 05:43 PM