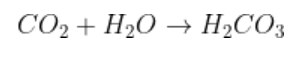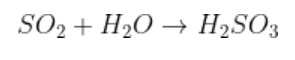1. Why acid anhydrides are so reactive in nature?
Here, electron donation to one carbonyl group competes with the electron donation to the second carbonyl group. Thus, they are less stable and reactive in nature.
2. What are the examples of acid anhydrides?
Sulfuric acid, Calcium oxide are some examples of acid anhydrides.
3. What does acid anhydride mean?
A compound acid that is formed by the removal of water is known as acid anhydride.
4. What intermolecular forces are present in liquid ethanoic anhydride?
In liquid ethanoic anhydride, both dipole-dipole and van der Waals depression forces are present.
5. What is the difference between a symmetrical and an unsymmetrical acid anhydride?
A symmetrical acid anhydride is formed from two identical carboxylic acid molecules, while an unsymmetrical acid anhydride is formed from two different carboxylic acid molecules. Symmetrical anhydrides have identical R groups on both sides of the oxygen bridge.
6. How do acid anhydrides contribute to the synthesis of aspirin?
Acetic anhydride is used in the synthesis of aspirin (acetylsalicylic acid). It reacts with salicylic acid to introduce the acetyl group, forming aspirin and acetic acid as a byproduct.
7. What is the role of acid anhydrides in the food industry?
Some acid anhydrides, like acetic anhydride, are used in food processing as acetylating agents. They can modify starches and other food components to alter properties like viscosity and stability.
8. How do acid anhydrides react with ammonia?
Acid anhydrides react with ammonia to form amides. One molecule of the anhydride reacts with two molecules of ammonia to produce two molecules of the corresponding amide.
9. How can you distinguish between an acid anhydride and a carboxylic acid in the laboratory?
Acid anhydrides can be distinguished from carboxylic acids by their reaction with water. Anhydrides react vigorously with water to form carboxylic acids, while carboxylic acids do not. Additionally, anhydrides do not typically show the broad O-H stretch in IR spectroscopy that is characteristic of carboxylic acids.
10. What is the IUPAC naming convention for acid anhydrides?
Acid anhydrides are named by adding the word "anhydride" after the name of the corresponding carboxylic acid. For example, the anhydride of acetic acid is called acetic anhydride.
11. What is the role of acid anhydrides in the synthesis of polyimides?
Acid anhydrides, particularly aromatic dianhydrides, are key components in the synthesis of polyimides. They react with diamines to form high-performance polymers with excellent thermal stability and mechanical properties.
12. What is the significance of acid anhydrides in the production of alkyd resins?
Acid anhydrides, such as phthalic anhydride, are crucial components in the synthesis of alkyd resins. They react with polyols and fatty acids to form the backbone of these important coating materials.
13. How can you synthesize an acid anhydride from a carboxylic acid?
Acid anhydrides can be synthesized by dehydrating two molecules of a carboxylic acid using a dehydrating agent like phosphorus pentoxide (P2O5) or by heating the acid with a dehydrating agent.
14. What is the mechanism of acid anhydride formation from carboxylic acids?
The mechanism involves the nucleophilic attack of one carboxylic acid molecule on another, followed by the elimination of water. This can be facilitated by heat or a dehydrating agent, which removes the water molecule and drives the reaction forward.
15. What is the significance of intramolecular anhydride formation in certain organic reactions?
Intramolecular anhydride formation can occur in dicarboxylic acids, leading to cyclic anhydrides. This process is important in the synthesis of certain heterocyclic compounds and in understanding the behavior of some natural products.
16. Why are acid anhydrides considered acid derivatives?
Acid anhydrides are considered acid derivatives because they can be derived from carboxylic acids and can be converted back to carboxylic acids through hydrolysis. They share similar reactivity patterns with other acid derivatives like acid chlorides and esters.
17. What is the significance of anhydride exchange reactions?
Anhydride exchange reactions involve the interchange of acyl groups between different anhydrides. This process is important in organic synthesis for creating mixed anhydrides and in understanding the equilibrium behavior of these compounds.
18. How does the structure of an acid anhydride differ from a carboxylic acid?
An acid anhydride has two acyl groups (RCO-) connected by an oxygen atom, while a carboxylic acid has one acyl group and a hydroxyl group (-OH). The anhydride lacks the -OH group present in carboxylic acids.
19. Why are acid anhydrides more reactive than carboxylic acids?
Acid anhydrides are more reactive because they have a strained, less stable structure compared to carboxylic acids. The oxygen bridge between the two carbonyl groups is easily cleaved, making anhydrides more susceptible to nucleophilic attack.
20. What happens when an acid anhydride reacts with water?
When an acid anhydride reacts with water, it undergoes hydrolysis to form two molecules of the corresponding carboxylic acid. This reaction is the reverse of the dehydration process used to form the anhydride.
21. How do acid anhydrides react with alcohols?
Acid anhydrides react with alcohols to form esters. One molecule of the anhydride reacts with one molecule of alcohol to produce one molecule of ester and one molecule of carboxylic acid.
22. What is an acid anhydride?
An acid anhydride is a compound formed by removing a water molecule from two carboxylic acid molecules. It contains two acyl groups bonded to a single oxygen atom. The general formula for an acid anhydride is (RCO)2O, where R represents an alkyl or aryl group.
23. What is the importance of acid anhydrides in organic synthesis?
Acid anhydrides are important in organic synthesis because they serve as acylating agents, allowing the introduction of acyl groups into various compounds. They are used in the synthesis of esters, amides, and other important organic compounds.
24. How do acid anhydrides react with primary amines?
Acid anhydrides react with primary amines to form N-substituted amides. One molecule of the anhydride reacts with one molecule of the primary amine to produce one molecule of N-substituted amide and one molecule of carboxylic acid.
25. What is the role of catalysts in acid anhydride reactions?
Catalysts, such as pyridine or 4-dimethylaminopyridine (DMAP), can accelerate acid anhydride reactions by activating the carbonyl group and making it more susceptible to nucleophilic attack. This increases the reaction rate and efficiency.
26. How does the reactivity of acid anhydrides compare to acid chlorides?
Acid anhydrides are generally less reactive than acid chlorides but more reactive than esters or amides. This intermediate reactivity makes them useful in situations where a milder acylating agent is needed compared to acid chlorides.
27. What is the significance of cyclic acid anhydrides?
Cyclic acid anhydrides, such as maleic anhydride or phthalic anhydride, are important in polymer chemistry and organic synthesis. They have unique reactivity due to their ring structure and are used in the production of polyester resins and other materials.
28. How do acid anhydrides react with Grignard reagents?
Acid anhydrides react with Grignard reagents to form ketones. The Grignard reagent attacks one of the carbonyl groups, leading to the formation of a ketone and a carboxylate salt.
29. What is the environmental impact of acid anhydrides?
Many acid anhydrides are irritants and can be harmful if inhaled or in contact with skin. They can react with moisture in the air to form acids, which can be corrosive. Proper handling and disposal are important to minimize environmental impact.
30. How do acid anhydrides react with inorganic bases like sodium hydroxide?
Acid anhydrides react with inorganic bases like sodium hydroxide to form two equivalents of the corresponding carboxylate salt. This reaction is often rapid and exothermic.
31. What is the importance of mixed anhydrides in biochemistry?
Mixed anhydrides, particularly those involving phosphoric acid, play crucial roles in biochemical processes. For example, the high-energy phosphoanhydride bonds in ATP drive many cellular reactions.
32. How does the presence of electron-withdrawing or electron-donating groups affect the reactivity of acid anhydrides?
Electron-withdrawing groups increase the electrophilicity of the carbonyl carbon, making the anhydride more reactive towards nucleophiles. Conversely, electron-donating groups decrease reactivity by making the carbonyl carbon less electrophilic.
33. How do acid anhydrides participate in Friedel-Crafts acylation reactions?
Acid anhydrides can serve as acylating agents in Friedel-Crafts reactions, introducing acyl groups onto aromatic rings. This reaction typically requires a Lewis acid catalyst like AlCl3 and produces a ketone product.
34. What is the role of acid anhydrides in polymer chemistry?
Acid anhydrides are used in the synthesis of polyesters and other polymers. They can react with diols to form polyester chains or with diamines to form polyamides. Cyclic anhydrides are particularly useful in creating cross-linked polymer networks.
35. How do acid anhydrides react with hydrogen halides?
Acid anhydrides react with hydrogen halides (HX) to form an acid halide and a carboxylic acid. This reaction can be used to synthesize acid chlorides, which are even more reactive than anhydrides.
36. How do acid anhydrides contribute to the formation of peptide bonds in protein synthesis?
While not commonly used in biological systems, acid anhydrides can form peptide bonds by reacting with amino acids. This reaction is more relevant in synthetic peptide chemistry, where activated forms of carboxylic acids (similar to anhydrides) are used to form peptide bonds.
37. What is the role of acid anhydrides in the production of artificial sweeteners?
Some artificial sweeteners, like sucralose, involve the use of acid anhydrides in their synthesis. For example, sucralose production involves the selective chlorination of sucrose using a combination of acid anhydrides and chlorinating agents.
38. How do acid anhydrides interact with enzymes in biological systems?
Acid anhydrides can react with nucleophilic groups in enzymes, such as the -OH group of serine or the -SH group of cysteine. This can lead to enzyme inhibition or modification, which is sometimes exploited in biochemical studies or drug design.
39. What is the significance of acid anhydrides in the synthesis of pharmaceuticals?
Acid anhydrides are used in the synthesis of various pharmaceuticals, often as acylating agents. They can be used to modify drug molecules, introduce protecting groups, or create prodrugs with improved pharmacokinetic properties.
40. How do acid anhydrides contribute to the production of biodiesel?
While not directly used in biodiesel production, acid anhydrides can be used to modify vegetable oils or animal fats to improve their properties as biodiesel. For example, maleic anhydride can be used to increase the oxidative stability of biodiesel.
41. What is the role of acid anhydrides in the synthesis of dyes?
Acid anhydrides are used in the synthesis of certain dyes, particularly those involving the introduction of acyl groups. For example, phthalic anhydride is used in the production of fluorescein and related dyes.
42. How do acid anhydrides react with organometallic compounds other than Grignard reagents?
Acid anhydrides can react with various organometallic compounds, such as organolithium or organocuprate reagents. These reactions typically result in the formation of ketones, similar to the reaction with Grignard reagents.
43. What is the importance of acid anhydrides in the production of plasticizers?
Acid anhydrides, particularly phthalic anhydride, are key components in the production of plasticizers like phthalate esters. These compounds are used to increase the flexibility and durability of plastics.
44. How do acid anhydrides participate in Diels-Alder reactions?
Some cyclic acid anhydrides, like maleic anhydride, can act as dienophiles in Diels-Alder reactions. This reactivity is used to synthesize complex cyclic compounds and in polymer chemistry for creating cross-linked materials.
45. What is the role of acid anhydrides in the synthesis of lactones?
Acid anhydrides can react intramolecularly with hydroxyl groups on the same molecule to form lactones. This process is important in the synthesis of certain natural products and pharmaceuticals.
46. How do acid anhydrides contribute to the production of surfactants?
Acid anhydrides are used in the synthesis of certain surfactants, particularly those involving the introduction of hydrophobic groups. For example, succinic anhydride can be used to modify hydrophilic compounds to create amphiphilic surfactants.
47. What is the significance of acid anhydrides in green chemistry?
While many traditional acid anhydrides are not considered green, some bio-based anhydrides derived from renewable resources are being explored as more sustainable alternatives in various chemical processes.
48. How do acid anhydrides react with thiols?
Acid anhydrides react with thiols to form thioesters. This reaction is similar to the reaction with alcohols but often proceeds more rapidly due to the higher nucleophilicity of sulfur compared to oxygen.
49. What is the role of acid anhydrides in the production of flame retardants?
Some acid anhydrides, particularly those containing phosphorus or halogens, are used in the synthesis of flame retardant compounds. These can be incorporated into polymers or applied as coatings to improve fire resistance.
50. How do acid anhydrides contribute to the synthesis of photoresists used in semiconductor manufacturing?
Acid anhydrides are used in the synthesis of certain photoresist polymers. They can introduce cross-linkable groups or modify the solubility properties of the photoresist, which is crucial for the photolithography process in semiconductor manufacturing.
51. What is the significance of acid anhydrides in the production of epoxy resins?
Acid anhydrides, such as phthalic anhydride or hexahydrophthalic anhydride, are used as curing agents for epoxy resins. They react with the epoxide groups to form a cross-linked network, imparting hardness and chemical resistance to the final product.
52. How do acid anhydrides interact with amino acids in non-enzymatic browning reactions?
In food chemistry, acid anhydrides can participate in Maillard reactions (non-enzymatic browning) by reacting with amino acids. This can contribute to flavor development and color changes in certain food products during processing or cooking.
53. How do acid anhydrides contribute to the modification of cellulose and other polysaccharides?
Acid anhydrides can be used to acetylate cellulose and other polysaccharides, modifying their properties. This process is used in the production of cellulose acetate and other modified natural polymers with applications in textiles and plastics.
54. How do acid anhydrides participate in the synthesis of cyclic imides?
Acid anhydrides react with primary amines to form cyclic imides through a two-step process involving initial amide formation followed by cyclization. This reaction is important in the synthesis of various heterocyclic compounds and pharmaceuticals.