Schiff Reagent and Test - Composition, Preparation, Structure, Reaction, FAQs
Ever think about how chemists detect unknown compounds or the presence of certain molecules in solutions or medicines with the help of just a few drops? It seems difficult, but it is not, we have various reagents that help to detect the presence of certain groups or molecules in a solution. Out of all these reagents, we are going to discuss Schiff’s reagent. It is used to detect the presence of an aldehyde group in organic compounds.
- What Is The Schiff’s Test?
- Schiff's Reagent
- Composition Of Schiff's Reagent
- Schiff’s's Reagent Preparation
- Rosaniline Structure
- Schiff’s Test Reaction
- Some Solved Examples

Schiff’s reagent was invented by Hugo Schiff’s, a German chemist. The Schiff’s test is performed with the help of Schiff’s reagent. It is a colourless or pale solution of fuchsin dye treated with sulphurous acid. When Schiff's reagent comes into contact with an aldehyde, it changes its colour from colourless to pink. This test is simple yet widely applicable in various industries like food testing, forensic science, industrial quality control, etc. In this article, we will read about what is Schiff's reagent and test questions related to Schiff’s reagent and test. Scroll down to know more.
What Is The Schiff’s Test?
This qualitative test for aldehydes (used to check the presence of aldehydes) is performed using Schiff’s reagent. The sample that needs to be examined is combined with Schiff’s reagent. If aldehyde is present, a characteristic magenta-pink color is obtained.
Any compound with an aldehydic -CHO group will show a positive Schiff’s test. Example: Human skin is stained due to the presence of aldehydes in the tissues of the skin. Although sometimes aliphatic ketones might show positive results, the overall process for colorization is slow and takes time for the pink color to emerge. Aromatic aldehyde reacts similarly to aliphatic ketones with Schiff’s reagent, but aromatic ketones do not produce color in the presence of Schiff’s reagent.
Schiff's Reagent
The name of Schiff’s reagent is Fuchsine or basic fuchsine. The IUPAC name of Schiff's reagent is 4-[((4-Dimethylaminophenyl)imino)methyl]phenol. Schiff’s reagent is a product of the formulation of certain dyes, such as fuchsin and sodium bisulfate, which chemically react with aldehydes to form a bright pink product. Schiff’s reagent is sometimes referred to as leucofuchsin. The term ‘leuco’ means white or absence of color, which is termed due to a very pale yellow color or nearly colorless solution. The fuchsin dye is decolorized by the addition of sulfurous acid (or its conjugate base bisulfate) due to which it is also referred to as fuchsin-sulfurous acid. Fuchsin dye is also known as rosaniline hydrochloride and is marked by its magenta color.
Composition Of Schiff's Reagent
The Schiff’s reagent is prepared by using fuchsin (<1%) dye in water (>98%) combined with sodium bisulfite (<1%) dissolved in a solution of hydrochloric acid (<1%). The solution is shaken at intervals, followed by decolorization with charcoal. The mixture is then filtered. Fresh activated charcoal must be used to ensure the formation of a perfectly colorless solution. If the solution is not colorless, the solution is refiltered.
Schiff’s's Reagent Preparation
The precise steps of preparation are stated below:
- In 900 mL of boiling water, dissolve 5g basic fuchsin.
- Cool down the solution until it reaches around 50°C.
- Slowly add 100 mL of 1M HCl to the dilute solution of fuchsin.
- Again, cool down the solution to 25°C.
- To the cooled solution, add 10g K2S2O5.
- Shake the above solution for 3 minutes and leave the solution mixture to incubate in a dark room for 24 hours.
- After incubation and resting, add 5g of fine activated charcoal to the reaction mixture.
- Shake the solution for 3 minutes and filter.
- A crystal clear solution must be obtained, or else refiltration and retreatment must be done.
- Store the prepared solution at 4°C in a bottle covered with foil. Schiff’s reagent precipitates a white crystalline substance if not stored properly. Thus, fresh batches at intervals of 2-3 weeks must be prepared to ensure accurate results.
| Related topics link, |
Rosaniline Structure
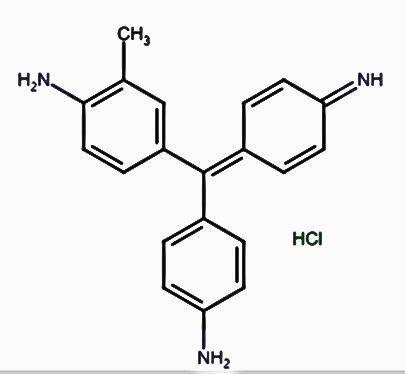
Rosaniline hydrochloride or fuchsin is a pinkish magenta color dye used in Schiff’s reagent to detect the presence of aldehydes. In Schiff’s reagent, it is decolorized by sulfurous acid to form a colorless solution.
Schiff’s Reagent formula: Schiff’s reagent’s molecular formula is C20H19N3·HCl.
Schiff’s Test Reaction
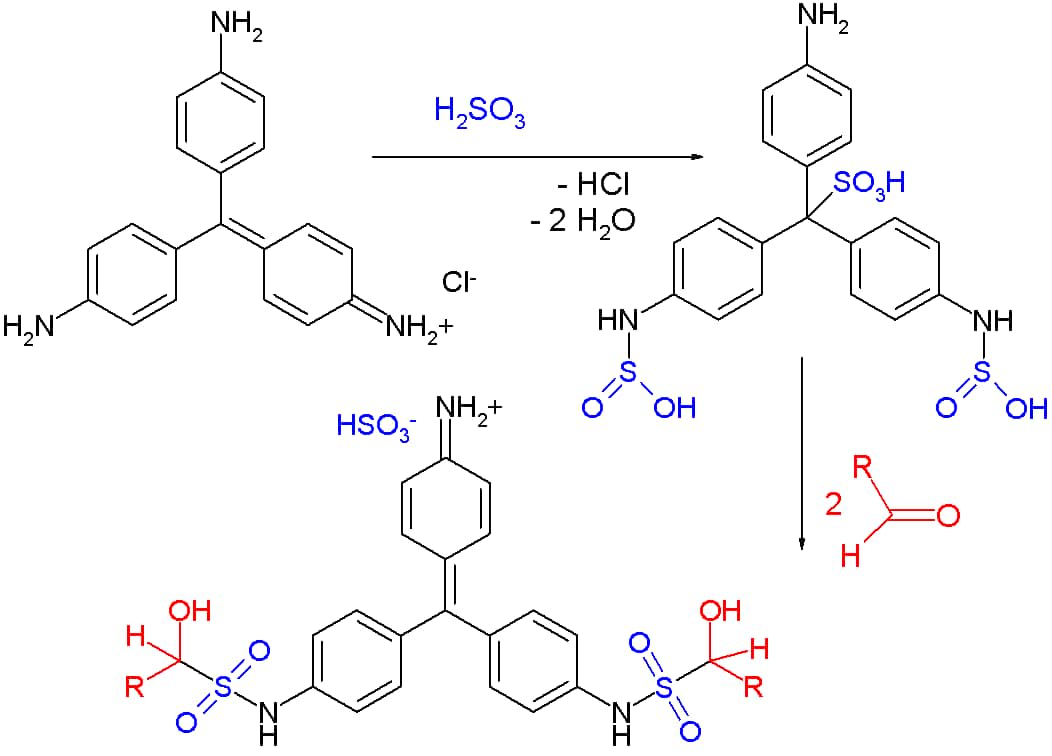
The reaction is initiated when rosaniline hydrochloride or fuchsin, with a characteristic magenta color, is decolorized by adding sulfurous acid H2SO3. The decolorization is due to the distortion of the chromophore (chromophore is a term used to define atoms or groups of atoms that are responsible for the presence of color in a compound) by the addition of the sulphonic acid -SO3H group to the central carbon, resulting in loss of the quinoid ring.
This is known as Schiff’s reagent. When this new sulphonic acid substituted compound or Schiff’s reagent reacts with two molecules of aldehydes, condensation takes place which reforms the chromophore and restores the quinoid ring. Thus, a pink-magenta color is reproduced, indicating the presence of aldehydes.
Some Solved Examples
Question.1 What is Schiff's reagent primarily used to detect?
a) Alcohols
b) Aldehydes
c) Ketones
d) Carboxylic acids
Solution:
Schiff’s test is a chemical test used to check for the presence of aldehydes in a given analyte.
Hence , the correct answer is option b) Aldehydes
Question.2 What is the composition of Schiff’s reagent?
a) Fuchsine + Sulfuric acid
b) Fuchsine + Sulfur dioxide
c) Fuchsine + Sodium hydroxide
d) Fuchsine + Acetic acid
Solution: Schiff's reagent is a solution used primarily for detecting aldehydes. It is composed of the Fuchsine (or Basic Fuchsine) and Sulfur Dioxide (SO₂)
Hence , the correct answer is option b) Fuchsine + Sulfur dioxide
Practice more questions with the link given below:
| Methods of Preparation of Aldehydes and Ketones Practice Questions and MCQ |
| Reaction of Aldehydes and Ketones practice questions and MCQ |
Also Read:
Frequently Asked Questions (FAQs)
Schiff reagent is a chemical solution that is used to detect the presence of an aldehyde group in organic compounds. It is a solution of fuchsin dye treated with sulphurous acid.
Schiff Reagent is used in laboratory testing to identify and visualise aldehyde functional groups within various biological samples, such as tissues or carbohydrates. This is particularly useful in histological staining.
There are a few limitations and considerations while using Schiff's reagent
- One primary concern is that it can produce false positives if other substances that contain reducing agents are present, as they may react similarly.
- Proper handling and storage of the reagent are essential, as it can degrade or lose effectiveness over time.
- The staining needs to be interpreted cautiously
A variety of samples can be tested using Schiff reagent:
- Biological tissues
- Food products
- Detection of polysaccharide
Schiff Reagent specifically detects aldehyde groups, whereas other tests, such as Benedict's test or Fehling's test, evaluate reducing sugars and may detect both aldehydes and certain ketones. Schiff’s test is unique in its distinct magenta colour change associated specifically with aldehyde detection and is particularly useful for certain types of carbohydrates.
The active component is a colorless sulfonic acid derivative of fuchsin. It has a central carbon atom bonded to three aromatic rings, with sulfonic acid groups (-SO3H) attached.
The reactivity of aldehydes with Schiff's reagent can vary based on their structure. Generally, aliphatic aldehydes react more readily than aromatic aldehydes. Steric hindrance around the carbonyl group can also affect reactivity.
When Schiff's reagent reacts with an aldehyde, the colorless sulfonic acid derivative is converted back to the original fuchsin dye structure. This involves the elimination of sulfurous acid and the formation of a new carbon-nitrogen double bond.
While Schiff's test can detect the presence of aldehydes, it cannot distinguish between different aldehydes on its own. Additional tests or analytical methods would be needed to identify specific aldehydes.
While Schiff's reagent is typically used for liquid-phase detection, it can be adapted for gas-phase aldehyde detection. This is sometimes done by impregnating filter paper with the reagent and exposing it to the gas.
Schiff's reagent is a chemical solution used to detect aldehydes. It consists of fuchsin (a magenta dye) that has been decolorized by sulfur dioxide. When it reacts with an aldehyde, it produces a characteristic magenta color.
Schiff's base is a compound formed by the reaction of an aldehyde or ketone with a primary amine. Schiff's reagent, on the other hand, is a specific solution used to detect aldehydes. They are different concepts despite the similar names.
Yes, Schiff's reagent can detect aldehyde groups in larger molecules like carbohydrates, particularly those with free aldehyde groups or those that can form aldehydes through ring-opening (e.g., glucose).
The PAS stain uses Schiff's reagent to detect aldehydes formed by the oxidation of certain carbohydrates with periodic acid. This technique is used to visualize glycogen, mucin, and other carbohydrate-containing structures in tissues.
Yes, Schiff's reagent can detect aldehydes in complex mixtures, but other components may interfere with the test. In such cases, separation techniques might be necessary before performing the test.
The main component of Schiff's reagent is para-rosaniline, also known as basic fuchsin or magenta II. Its chemical name is 4-[(4-aminophenyl)(4-imino-2,5-cyclohexadien-1-ylidene)methyl]-2-methylaniline hydrochloride.
Ketones generally don't react with Schiff's reagent because they lack the hydrogen atom attached to the carbonyl carbon, which is crucial for the reaction mechanism. This structural difference makes ketones less reactive under standard conditions.
While both Schiff's and Tollens' reagents detect aldehydes, they work differently. Schiff's test produces a color change, while Tollens' test produces a silver mirror. Schiff's test is generally more sensitive but less specific than Tollens' test.
Yes, environmental factors like temperature, light exposure, and air oxidation can affect Schiff's reagent and potentially influence test results. Proper storage and handling are essential for accurate results.
Yes, Schiff's reagent can detect aldehydes in biological samples. This is particularly useful in histology and cytology for visualizing certain cellular structures and components that contain or can form aldehydes.
Schiff's reagent is prepared by dissolving fuchsin (rosaniline hydrochloride) in water, then adding sodium bisulfite and concentrated hydrochloric acid. This process decolorizes the solution, which becomes colorless or pale yellow.
The sulfur dioxide (from sodium bisulfite and hydrochloric acid) reduces the fuchsin dye, forming a colorless sulfonic acid derivative. This colorless compound is what reacts with aldehydes to produce the magenta color.
Schiff's reagent contains sulfur dioxide, which can be harmful if inhaled. It should be handled in a well-ventilated area. Also, it's light-sensitive, so it should be stored in a dark bottle and protected from light.
Properly stored Schiff's reagent can last for several months. However, it's sensitive to light and air exposure, which can cause it to deteriorate. Regular quality checks are recommended to ensure its effectiveness.
Sulfur dioxide is crucial in preparing Schiff's reagent. It reduces the fuchsin dye to its colorless form (the sulfonic acid derivative). This colorless compound is what reacts with aldehydes to regenerate the colored form.
Schiff's reagent primarily reacts with aldehydes, producing a magenta color. Most ketones do not react with Schiff's reagent under normal conditions, allowing it to distinguish between these two types of carbonyl compounds.
Schiff's test is qualitative because it indicates the presence or absence of aldehydes based on color change, but it doesn't provide quantitative information about the amount of aldehyde present.
Higher temperatures generally accelerate the reaction between Schiff's reagent and aldehydes. However, excessive heat can lead to false positives or decomposition of the reagent, so room temperature is typically recommended.
False positives can occur due to the presence of certain compounds that can regenerate the fuchsin dye, such as strong oxidizing agents. Additionally, some ketones may react under certain conditions, leading to false positives.
The Schiff's test is typically performed in acidic conditions. Very high or low pH can affect the reaction, potentially leading to false results. The optimal pH range is usually between 1 and 3.
The aldehyde reacts with the sulfonic acid derivative in Schiff's reagent, eliminating sulfurous acid. This regenerates the original fuchsin dye structure, resulting in the characteristic magenta color.
Schiff's reagent can detect most aldehydes, but some exceptions exist. For example, aromatic aldehydes like benzaldehyde may react slowly or not at all under standard conditions.
The reaction time can vary depending on the specific aldehyde and conditions, but typically, a color change is observed within a few minutes. Some aldehydes may react more slowly, taking up to 30 minutes.
The magenta color indicates the regeneration of the fuchsin dye structure, which only occurs in the presence of aldehydes. This color change is the key indicator of a positive test for aldehydes.
While primarily a qualitative test, Schiff's reagent can be used for semi-quantitative analysis through colorimetry. The intensity of the magenta color is roughly proportional to the aldehyde concentration, allowing for approximate quantification.
Generally, a higher concentration of aldehyde will produce a more intense magenta color. However, the relationship is not perfectly linear, especially at very high concentrations where the color may saturate.
Schiff's test is used in organic chemistry to detect aldehydes, in biochemistry to analyze carbohydrates, and in histology (as part of the PAS stain) to visualize certain cellular components. It's also used in forensic science to detect formaldehyde.
A weak positive result typically shows a pale pink or light magenta color, indicating a low concentration of aldehydes. A strong positive result shows a deep magenta color, suggesting a higher concentration of aldehydes.
Strong reducing agents can interfere with Schiff's test by reducing the regenerated fuchsin dye back to its colorless form, potentially leading to false negative results. Care should be taken to avoid such interfering substances.
Yes, Schiff's reagent can be used to detect aldehydes in air or environmental samples. This is often done by bubbling air through a solution of the reagent or using reagent-impregnated papers or gels.
Aliphatic aldehydes generally react more readily with Schiff's reagent compared to aromatic aldehydes. This is due to the electron-withdrawing effect of the aromatic ring, which makes the carbonyl group less reactive.
Hydrochloric acid serves two purposes in Schiff's reagent preparation: it provides an acidic environment necessary for the reaction, and it helps generate sulfur dioxide from sodium bisulfite, which is crucial for decolorizing the fuchsin dye.
To confirm a positive Schiff's test, you can perform additional tests specific for aldehydes, such as Tollens' test or Fehling's test. You can also use analytical techniques like HPLC or GC-MS for definitive identification.
The selectivity is based on the reaction mechanism. Aldehydes have a hydrogen attached to the carbonyl carbon, allowing for the formation of an unstable intermediate that can eliminate sulfurous acid and regenerate the colored dye. Ketones lack this hydrogen, making this mechanism unfavorable.
Electron-withdrawing groups generally increase the reactivity of aldehydes with Schiff's reagent by making the carbonyl carbon more electrophilic. Conversely, electron-donating groups tend to decrease reactivity by making the carbonyl carbon less electrophilic.
While Schiff's reagent is typically used in aqueous solutions, it can be adapted for use in some non-aqueous solvents. However, the reactivity and color development may differ from aqueous conditions, and the results should be interpreted cautiously.
Generally, the chain length of aliphatic aldehydes has a minimal effect on their reactivity with Schiff's reagent. However, very long-chain aldehydes might react more slowly due to decreased solubility and increased steric hindrance.
Schiff's reagent is used in the Feulgen stain, a histochemical technique for visualizing DNA in cell nuclei. The process involves hydrolyzing DNA to create free aldehyde groups, which then react with Schiff's reagent, allowing for DNA quantification and localization.
Modifications to improve Schiff's reagent include using different dyes or adding catalysts to enhance reactivity. Some variations use p-rosaniline instead of basic fuchsin, or add compounds like sodium metabisulfite to stabilize the reagent.
While not directly related, both involve reactions with carbonyl compounds. Schiff's reagent detects aldehydes through a specific color-forming reaction, while Schiff base formation is a more general condensation reaction between amines and carbonyl compounds.
Conjugation in an aldehyde can affect its reactivity with Schiff's reagent. Conjugated aldehydes, like cinnamaldehyde, may react more slowly due to the delocalization of electrons, which can stabilize the carbonyl group and reduce its reactivity.
Yes, Schiff's reagent can detect aldehyde groups in polymers or macromolecules, particularly those with accessible aldehyde groups. This is useful in analyzing certain polysaccharides, modified proteins, or polymers with aldehyde end groups.
Other functional groups can influence the Schiff's test. For example, strongly electron-withdrawing groups near the aldehyde can enhance reactivity, while bulky groups might hinder it. Some groups may also interfere with the test or cause side reactions.
Common sources of error include using degraded or improperly stored Schiff's reagent, contamination of samples or equipment, incorrect pH, temperature variations, and the presence of interfering substances. Proper technique and fresh reagents are crucial for accurate results.
For high-throughput screening, the Schiff's test can be miniaturized and automated using microplate readers or flow injection analysis systems. The color change can be quantified spectrophotometrically, allowing for rapid analysis of multiple samples simultaneously.
Also Read
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:26 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:25 PM
02 Jul'25 05:12 PM
