Acids Bases and Salts - Definition, Types, Concept, FAQs
Have you ever wondered why some substances taste sour, some taste bitter, and some remain neutral? How do simple compounds like vinegar, baking soda, or common salt behave so differently in water? You will get these answers by reading this article on acids, bases and salts. Acids are known for their sour taste and their reaction with metals, bases are bitter and slippery, while salts are products of their neutralisation.
- Acid
- Bases
- Salts
- Salt is an Acid or Base
- Discovery of Acids, Bases and Salts
- Lux-Flood Concept
- Difference Between Acids, Bases and Salts
- Some Solved Examples
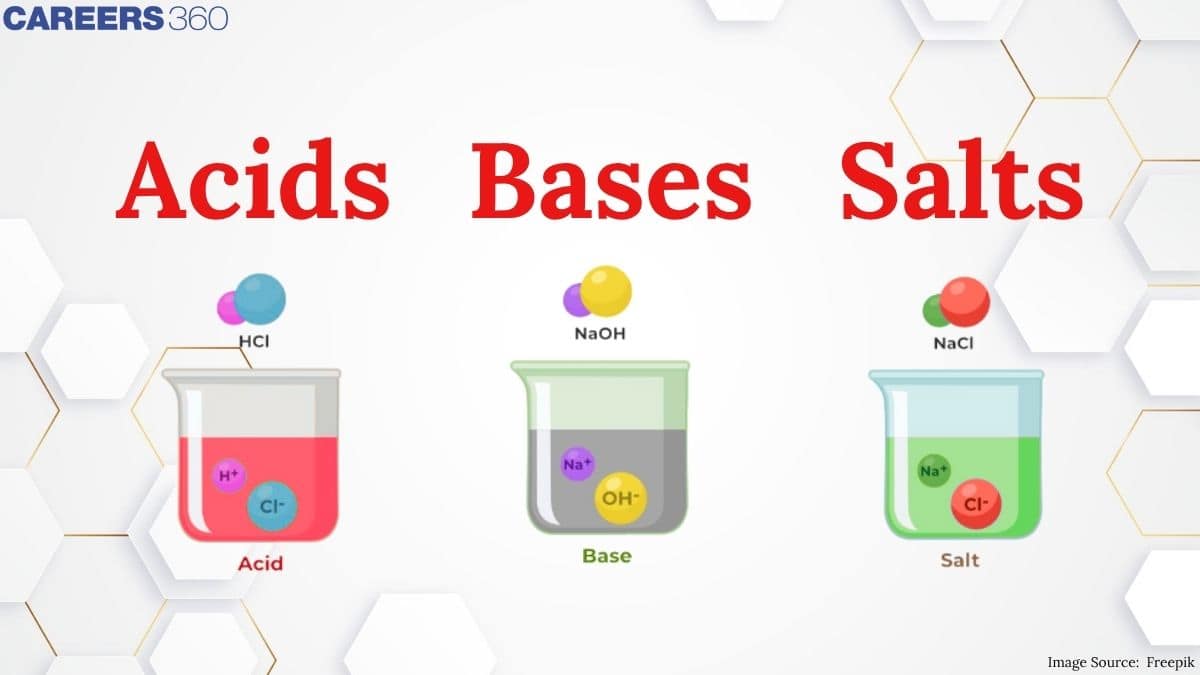
In this article, we will read about the acid-base and their discovery, concepts and some solved examples and reaction related to them.
Acid
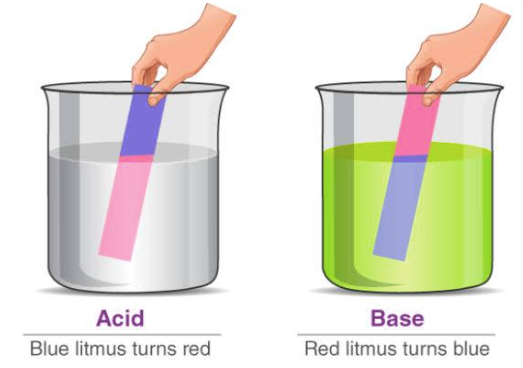
The word acid is derived from the word acidus meaning sour to taste. Acidic chemical compounds are known to give a sour or acidic taste when allowed to dissolve in water. An acid can remain energetically favourable even after loss of a hydrogen ion from its compound. Acids turn blue litmus red. The reason for this output s that the pigment in the litmus paper reacts with the H+ ions resulting in chemical changes where the chemical bonds are tuned to reflect light of longer wavelength making it appear red to our eyes. The reaction between the pigment in the litmus paper and the H+ ions of an acid leads to the absorption of blue to green wavelengths. Acids mainly oxidize other chemical compounds or simply change the colour of the substance. Acids can be either organic or inorganic, former containing a carboxyl group, a hydroxyl group and hydrogen atoms and the later contains a metal ion.
Bases
Bases are identified by their slippery texture and a bitter taste. Bases able to get dissolved in water are defined as alkali. Bases react with acids to produce salt and water and this chemical reaction is termed as neutralisation. Bases turn red litmus blue. The reason behind this colour change is explained as follows: The OH- ions or hydroxyl ions react differently with the pigment present in the litmus paper. It absorbs green and red wavelengths on reacting with the hydroxyl ion, reflecting short wavelengths to appear blue in colour. Bases are capable of changing the colour of the indicators. Phenolphthalein turns pink in the presence of a base.
Salts
Salts are produced as a result of a reaction between an acid and a base i.e. neutralisation reaction. Salts are described as ionic compounds composed of a cation and an anion, where a cation is other than H+ and an anion is other than OH-.Salt is a chemical species that can make water less acidic or take away the acidity of water. The best-known example of a salt is NaCl (sodium chloride).Acid-base salt water example
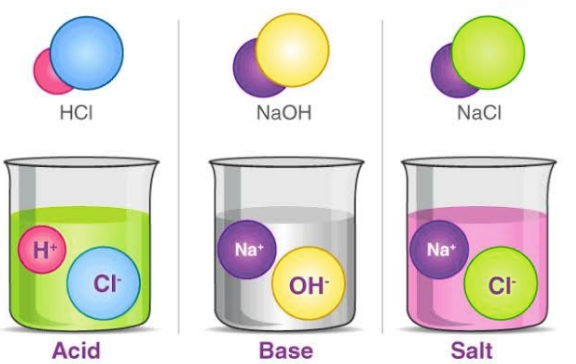
Salt is an Acid or Base
Salts can be of the following types-
-
Acidic salt
-
Basic/Alkali salt
-
Double salt
-
Mixed salt
Discovery of Acids, Bases and Salts
In 1663, Robert Boyle noticed a class of substances having sour taste, ability to change colour of vegetable dyes (indicators), tendency to react with certain metals to evolve hydrogen and high solvent power. These substances were called acids. The term base, was introduced by Roulle in 1754. Bases are regarded as chemical substances changing red litmus blue, slippery in nature, soapy to touch, neutralize acids to form salt amd water, tasted bitter. Salts are nothing but ionic compounds formed by the reaction between acid and base giving out water molecule. Salts can be organic or inorganic. The relative amount of ions present in the salt makes it neutral. After the discovery of oxygen, Lavoisier (1778) suggested that oxygen is essential component of all acids. This was disproved by Dave (1816). He showed that hydrochloric acid does not contain oxygen. Further, Leibig in 1838 defined acids are the substances having hydrogen atom or atoms replaceable by metals. There afterwards, concepts like Arrhenius, Bronsted-Lowry, Lewis and Lux-Flood etc were put forward.
Let us see one by one:-
Arrhenius Concept
According to him acid is defined as a substance that dissociates to produce hydrogen ions when dissolved in water.
Eg: HCl, H2SO4, SO3
$\mathrm{HCl}_{\text {gas }}+$ Water $\rightarrow \mathrm{H}^{+}{ }_{\mathrm{aq}}+\mathrm{Cl}^{-}{ }_{\mathrm{aq}}$
A base is defined as a substance that dissociates in water to produce hydroxyl ions.
Eg: NaOH, KOH, NH3
|
Related Topics Link, |
Bronsted-Lowry Concept
J.N. Bronsted and T.M. Lowry independently forwarded the concept of acids and bases in 1923. They defined acids as follows:
Acids are proton donors.
Eg: $\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}^{+}$
Bases are proton acceptors.
Eg: $\mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}^{+} \rightleftharpoons \mathrm{CH}_3 \mathrm{COOH}$
From above example, it is possible to say that when an acid loses proton, the residual part of it has a tendency to regain proton. Hence, it acts as a base. Thus, an acid and a base maybe therefore, defined by the general equation-
Acid ⇌ H++ Base
Lewis Concept
G.N Lewis in 1923 defined acid as an electron pair acceptor and base as a electron pair donor.
Lux-Flood Concept
The definitions proposed by H. Lux (1939) were extended by H. Flood (1947). They described acid-base behaviour in terms of the oxide ion.
The base is an oxide ion donor, while the acid is an oxide ion acceptor.
$\begin{aligned} & \mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3 \\ & \mathrm{CaO} \rightarrow \mathrm{Ca}\end{aligned}$
This concept has been extended to include transfer of anions like halide (X-), sulphide(S2-).
The bases are anion donor while acids are anion acceptors.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
Is Salt Acidic or Basic?
Salts can be acidic, basic or neutral.
NaCl, KCl are neutral salts because these salts are formed out of a strong acid and a strong base, which do not hydrolyse. The pH remains constant, i.e. neutral at 7. The cation doesn’t alter the H+ ion concentration, and the anion does not attract the H+ ion. Hence, the salt remains neutral. Acidic salts are formed as a product of a neutralisation reaction between a strong acid and a weak base.
E.g. – ammonium chloride NH4Cl
Basic salts are a result of reaction between Weak acids and a strong base. E.g. – sodium acetate (NaAc).
Difference Between Acids, Bases and Salts
- An acid is described as a chemical compound which makes the water solution sour to taste and turns blue litmus to red colour.
- A base is a chemical compound whose aqueous solution is bitter to taste. It turns red litmus blue in colour or is capable of neutralising acids.
- Table Salt, being a neutral chemical species, has nil effect on the litmus paper.
- E.g.: NaCl (sodium salt) is obtained as a product of the reaction between a strong acid - Hydrochloric acid (HCl), and a strong base - sodium hydroxide (NaOH). NaCl is a cluster of Na+ cations and Cl- anions.
- Acids can be naturally occurring or synthetic. They are very important I the world of chemistry because they allow us to separate otherwise chemically similar substances.
- Natural acids are organic compounds that contain hydrogen and oxygen. They can be classified into either strong or weak acids based on their extent of ionisation.
- Some naturally occurring acids are: Vinegar (acetic acid), citric acid, tartaric acid.
Also check-
Some Solved Examples
Question 1: According to Brosted $\mathrm{H}_2 \mathrm{O}$ behaves like
1) Bronsted Acid
2) Bronsted Base
3) (correct) Amphoteric
4) None of these
Solution:
As we learned from
Dual nature of $\mathrm{H}_2 \mathrm{O}$ -
Water ( $\mathrm{H}_2 \mathrm{O}$ ) can play the role of an acid as well as base.
- wherein
$
\mathrm{HCl}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{Cl}^{-}
$
$\mathrm{H}_2 \mathrm{O}$ act as base
$
\mathrm{H}_2 \mathrm{O}+\mathrm{NH}_3 \rightleftharpoons \mathrm{NH}_4^{+}+\mathrm{O} \overline{\mathrm{H}}
$
$\mathrm{H}_2 \mathrm{O}$ act as acid
With strong acid $\mathrm{H}_2 \mathrm{O}$ will release $\mathrm{H}^{+}$and strong base $\mathrm{H}_2 \mathrm{O}$ will gain $\mathrm{H}^{+}$
Hence, the answer is option (3).
Question 2:
Conjugate base of H2O will be :
1) (correct) OH-
2) H3O+
3) O2-
4) None of these
Solution:
As we learned from
Conjugate acid-base pair -
The acid-base pair that differ only by one proton is called a conjugate acid-base pair.
Bronsted acid
$-\mathrm{H}^{+} \rightarrow$ Conjugate base
$$
\mathrm{H}_2 \mathrm{O}-\mathrm{H}^{+} \rightarrow \mathrm{OH}^{-}
$$
Hence, the answer is option (1).
Question 3:
Correct pair of Lewis acid and Lewis base respectively.
1) NH3 , BF3
2) NH3, H2O
3) BF3 , BCl3
4) (correct) BF3 , NH3
Solution:
As we learned
Lewis acids and bases -
Lewis defined an acid as a species that accepts an electron pair and a base that donates an electron.
- wherein
In Lewis acid, many acids do not have protons.
$
\begin{aligned}
& \text { e.g. } \mathrm{BF}_3 \\
& \mathrm{BF}_3+\mathrm{NH}_3 \rightarrow B F_3: \mathrm{NH}_3
\end{aligned}
$
Hence, the answer is option (4).
Practice More Question With The Link Given Below
| Bronsted-Lowry and Lewis Acid-Base theory practice questions and MCQs |
| Ionisation Constant of Acids and Bases and pH of strong Acids and Bases practice questions and MCQs |
Frequently Asked Questions (FAQs)
Acids react with base to form salt and water and this reaction is called neutralization reaction.
According to Arrhenius concept, acids are classified as the substances wich dissociates into H+ ions when dissolved in water.
Table salt is neutral.
acid turns blue litmus red.
Base turns red litmus blue.
There will be no change in the litmus paper colour when neutral salt is used. Blue litmus paper turns red when acidic salt is used.
Soap being basic in nature turns red litmus blue.
Strong acids completely dissociate in water, releasing all their protons, while weak acids only partially dissociate. Strong acids have a lower pH and are more reactive than weak acids. Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
Conjugate acid-base pairs are related species that differ by a single proton. When an acid donates a proton, it becomes its conjugate base. When a base accepts a proton, it becomes its conjugate acid. For example, HCl (acid) and Cl- (conjugate base) form a conjugate acid-base pair.
A salt is an ionic compound formed by the neutralization reaction between an acid and a base. It consists of a positive ion (cation) from the base and a negative ion (anion) from the acid. Common table salt (NaCl) is an example of a salt formed from the reaction of HCl and NaOH.
The strength of acids and bases is measured using the pH scale, which ranges from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is basic. The lower the pH, the stronger the acid; the higher the pH, the stronger the base.
Indicators are substances that change color at specific pH values. They work by accepting or donating protons, which alters their molecular structure and, consequently, their color. This color change helps identify whether a solution is acidic, neutral, or basic.
The Arrhenius definition states that acids produce H+ ions in water, while bases produce OH- ions. The Brønsted-Lowry definition is broader, defining acids as proton donors and bases as proton acceptors, which applies to non-aqueous solutions and allows for more types of acid-base reactions.
Amphoteric substances can act as both acids and bases, depending on the reaction conditions. They can donate protons in some reactions (acting as an acid) and accept protons in others (acting as a base). Water is a common example of an amphoteric substance.
Autoionization of water is the process where water molecules react with each other to produce hydronium (H3O+) and hydroxide (OH-) ions. This reaction is represented as 2H2O ⇌ H3O+ + OH-. The product of these ion concentrations ([H3O+][OH-]) is always 10^-14 at 25°C, known as the ion product of water.
A buffer solution is a mixture that resists changes in pH when small amounts of acid or base are added. It typically consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers are crucial in maintaining stable pH levels in biological systems.
Acid rain forms when sulfur dioxide and nitrogen oxides, often from industrial emissions, react with water, oxygen, and other chemicals in the atmosphere. This creates acidic precipitation that can harm ecosystems, corrode buildings, and affect soil and water quality.
The common ion effect occurs when an ion from the dissociation of a weak acid or base is also present from another source. This additional presence suppresses the dissociation of the weak acid or base, effectively making it even weaker. This principle is used in creating buffer solutions.
The pH of a salt solution depends on whether the salt is formed from a strong or weak acid/base. Salts of strong acids and strong bases (like NaCl) are neutral. Salts of weak acids and strong bases (like CH3COONa) are basic, while salts of strong acids and weak bases (like NH4Cl) are acidic. The process of hydrolysis determines the final pH.
Buffers in biological systems, such as blood, maintain a stable pH by resisting changes when small amounts of acids or bases are added. They consist of a weak acid and its conjugate base (or vice versa). When an acid is added, the conjugate base neutralizes it; when a base is added, the weak acid neutralizes it, thus minimizing pH changes.
Amino acids have both acidic (-COOH) and basic (-NH2) groups, making them amphoteric. Their behavior depends on the pH of the solution. At low pH, they act as bases; at high pH, they act as acids. At their isoelectric point, they exist as zwitterions, with both positive and negative charges, affecting their solubility and reactivity.
Lewis acids and bases are defined based on electron pair interactions. A Lewis acid is an electron pair acceptor, while a Lewis base is an electron pair donor. This concept extends acid-base theory beyond proton transfer and applies to many reactions in organic and inorganic chemistry.
Acids are substances that donate protons (H+ ions) in aqueous solutions, while bases are substances that accept protons or release hydroxide ions (OH-) in aqueous solutions. This definition is known as the Brønsted-Lowry theory of acids and bases.
In a neutralization reaction, an acid and a base react to form water and a salt. The H+ ions from the acid combine with the OH- ions from the base to form water molecules. The remaining ions form the salt. For example: HCl + NaOH → H2O + NaCl.
pKa is the negative logarithm of Ka. A lower pKa value indicates a stronger acid because it means the acid dissociates more readily. Conversely, a higher pKa value indicates a weaker acid. This relationship allows for easy comparison of acid strengths across different compounds.
Polyprotic acids can donate more than one proton per molecule, while monoprotic acids donate only one. Polyprotic acids undergo stepwise dissociation, with each step having its own Ka value. Generally, the first dissociation is the strongest, with subsequent dissociations becoming progressively weaker.
Water plays a crucial role in acid-base chemistry as both a solvent and a reactant. It can act as both an acid and a base (amphoteric), allowing for proton transfer reactions. In aqueous solutions, acids and bases interact with water molecules, forming hydronium (H3O+) or hydroxide (OH-) ions.
The Henderson-Hasselbalch equation relates the pH of a buffer solution to the pKa of the weak acid and the concentrations of the acid and its conjugate base. It's expressed as pH = pKa + log([A-]/[HA]). This equation is crucial for calculating the pH of buffer solutions and understanding buffer behavior.
Acids and bases can act as catalysts in many reactions. They can facilitate proton transfer, making reactions proceed faster. For example, acid catalysis is crucial in ester hydrolysis and aldol condensations. Understanding acid-base catalysis is important in organic synthesis and biochemical processes.
Acid-base chemistry is crucial in environmental science, particularly in understanding and addressing issues like acid rain, ocean acidification, and soil pH. It affects the solubility of pollutants, the health of aquatic ecosystems, and the availability of nutrients in soil. Understanding acid-base interactions is key to developing environmental solutions.
Titration curves graphically represent the change in pH during an acid-base titration. The shape of the curve depends on the strength of the acid and base involved. Key features include the equivalence point (where acid and base have fully reacted) and the buffer region (where pH changes slowly). These curves are essential for understanding titration processes and selecting appropriate indicators.
The concept of hard and soft acids and bases (HSAB) extends Lewis acid-base theory. Hard acids and bases are small, highly charged species, while soft acids and bases are larger and more polarizable. This concept helps predict the stability of acid-base adducts and is useful in understanding various reactions in inorganic and organometallic chemistry.
Acid-base chemistry is crucial in food science for controlling flavor, texture, and preservation. pH affects the growth of microorganisms, enzyme activity, and chemical reactions in food. Acids are used as preservatives and flavor enhancers, while bases are used in food processing. Understanding acid-base interactions is essential for developing and improving food products.
Titration is a technique used to determine the concentration of an unknown acid or base by reacting it with a standard solution of known concentration. As the titrant is added, the pH changes, and the endpoint is reached when the acid and base have fully reacted. An indicator or pH meter is used to detect this endpoint.
Ka (acid dissociation constant) and Kb (base dissociation constant) are measures of the strength of acids and bases, respectively. They represent the extent to which an acid or base dissociates in water. A larger Ka indicates a stronger acid, while a larger Kb indicates a stronger base.
Organic acids contain carbon and are typically weaker than inorganic acids. They often have a carboxyl group (-COOH) as their acidic component. Inorganic acids do not contain carbon and are generally stronger. Organic acids are common in biological systems, while inorganic acids are more prevalent in industrial processes.
Temperature changes can shift acid-base equilibria. Generally, increasing temperature favors the endothermic direction of the reaction. For the autoionization of water, higher temperatures increase Kw (ion product of water), affecting the pH of neutral solutions. Temperature also affects the dissociation constants of acids and bases.
Lowry-Brønsted acid-base reactions can occur in non-aqueous solvents, where proton transfer takes place without water. The solvent often plays a role similar to water in aqueous solutions. Examples include reactions in liquid ammonia or acetic acid, where the solvent can act as an acid or base.
The leveling effect occurs when a solvent limits the strength of an acid or base. In water, for example, all strong acids are leveled to the strength of the hydronium ion (H3O+), and all strong bases are leveled to the strength of the hydroxide ion (OH-). This effect is important when considering reactions in different solvents.
pOH is the negative logarithm of hydroxide ion concentration, analogous to pH for hydrogen ions. In aqueous solutions at 25°C, pH + pOH = 14. pOH is particularly useful when dealing with basic solutions, as it directly relates to the concentration of hydroxide ions.
While traditional acid-base reactions involve proton transfer, the Lewis acid-base theory extends this concept to electron pair transfer. This broader definition allows acid-base chemistry to encompass many reactions typically classified as redox reactions, showing the interconnectedness of different types of chemical reactions.
Weak acids and bases only partially ionize in water, establishing an equilibrium between the unionized molecules and their ions. For a weak acid HA, the equilibrium is HA + H2O ⇌ H3O+ + A-. The extent of ionization is determined by the acid's Ka value. Similarly, for a weak base B, the equilibrium is B + H2O ⇌ BH+ + OH-.
Metal ions in solution can act as Lewis acids, accepting electron pairs from water or other ligands. This interaction can lead to the formation of complex ions and affect the acidity of the solution. For example, the hydrolysis of metal ions like Fe3+ can produce acidic solutions, while ions like Na+ typically do not significantly affect pH.
Ksp, the solubility product constant, is related to acid-base chemistry through the common ion effect and pH-dependent solubility. Many sparingly soluble salts involve ions that can act as acids or bases. The solubility of these compounds often depends on the pH of the solution, with acidic conditions typically increasing the solubility of basic salts and vice versa.
Acid-base reactions are fundamental in many analytical techniques. Titrations are used to determine the concentration of acids or bases in solutions. pH indicators and pH meters are essential tools in analytical chemistry. Acid-base properties also influence chromatographic separations and spectroscopic analyses of many compounds.
Blood pH is maintained around 7.4 by several buffer systems, primarily the bicarbonate buffer system (H2CO3/HCO3-). When acids enter the bloodstream, bicarbonate ions neutralize them, forming carbonic acid. When bases enter, carbonic acid neutralizes them, forming bicarbonate. This system, along with respiratory and renal mechanisms, helps maintain a narrow pH range essential for physiological processes.
The acid-base properties of drugs affect their absorption, distribution, metabolism, and excretion (ADME). The pH of different body compartments influences drug ionization, which in turn affects membrane permeability and drug distribution. Many drugs are weak acids or bases, and their effectiveness can depend on their state of ionization at physiological pH.
Proton affinity is the energy released when a proton (H+) is added to a base in the gas phase. It's a measure of the base strength in the absence of solvent effects. Higher proton affinity indicates a stronger base. This concept is important in understanding gas-phase acid-base reactions and in mass spectrometry.
Cleaning products often utilize acid-base chemistry. Acidic cleaners are used to remove mineral deposits and limescale, while basic cleaners are effective against organic stains and grease. Many detergents contain both acidic and basic components to tackle a variety of stains. Understanding the pH and chemical nature of stains helps in formulating effective cleaning products.
Zwitterions are molecules with both positive and negative charges, common in amino acids at their isoelectric point. They play crucial roles in protein structure and function. The zwitterionic nature of amino acids affects their solubility, reactivity, and ability to form peptide bonds. Understanding zwitterions is essential for comprehending protein behavior and many biochemical processes.
Soil pH, determined by the balance of acids and bases, greatly affects plant growth and nutrient availability. Acidic soils can increase the solubility of toxic metals and reduce the availability of essential nutrients. Basic soils can lead to nutrient deficiencies. Understanding and managing soil pH through liming or other treatments is crucial in agriculture and environmental management.
Acid-base reactions are fundamental in many battery systems. In lead-acid batteries, for example, the electrolyte is sulfuric acid, and the charging/discharging process involves acid-base reactions. In other battery types
Also Read
25 Aug'25 11:27 AM
25 Aug'25 09:49 AM
25 Aug'25 09:23 AM
22 Aug'25 06:01 PM
21 Aug'25 04:19 PM
20 Aug'25 05:06 PM
20 Aug'25 04:58 PM
18 Aug'25 12:59 PM
16 Aug'25 03:16 PM
02 Jul'25 07:41 PM