Mole Concept Basics - Definition, Formula, Examples, FAQs
To define the quantity of any substance in the field of chemistry, we use the term "mole". It is defined as the quantity comprising $6.023 \times 10^{23}$ elementary units. They can be atoms, molecules, ions, or electrons. The value mentioned is defined by the name as Avogadro's number, after the famous Italian scientist, Amedeo Avogadro. This number helps chemists calculate entities during a chemical reaction and relate the mass of the substance to the number of particles it contains. For instance, one mole of carbon would weigh 12 grams, which would have $6.023 \times 10^{23}$atoms of carbon.
This Story also Contains
- Understanding the Mole
- Relationship of Mole
- Determining the Total Number of Entities
- Some Solved Examples
- Conclusion

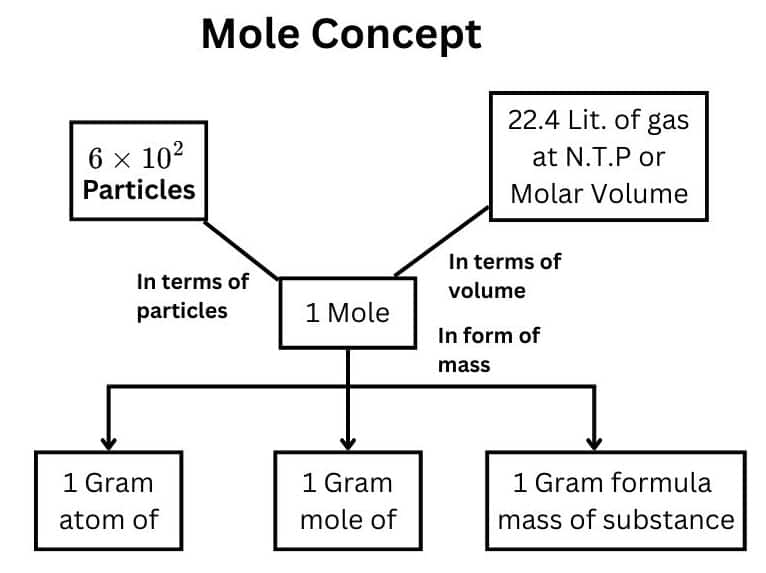
Understanding the Mole
- A mole is a unit that represents 6.023 x 1023 particles, atoms, molecules ions, etc., irrespective of their nature.
- Mole is related to the mass of the substance, the volume of the gaseous substance, and the number of particles
$6.023 \times 10^{23}$
where m = Wt. of substance in grams. and M = Molar mass of substance in G.m.m.
Here G.m.m = Gram molecular mass or molar mass, which is the mass of 1 mole of any substance
- Mole = (Volume of substance in litre )22.4 litre
- The volume of one mole of any gas is equal to 22.4 liters or dm3 at STP. It is known as molar volume.
- Mole = Number of identities Avogadro's number
Related Topics,
- Example Chemical Change
- Chemical Reactions
- Types of Chemical Reactions
- Physical Change Chemical Change
- Laws of Chemical Combination for Elements and Compounds
Relationship of Mole
A mole of any substance (like O2) stands for:
- $6.023 \times 10^{23}$ molecules of O2
- 2 x $6.023 \times 10^{23}$ atoms of Oxygen
- 32 gm of Oxygen
- 22.4 litre of O2 at STP.
Determining the Total Number of Entities
- Total number of molecules $=\operatorname{mole}(\mathrm{n}) \times \mathrm{N}_{\mathrm{A}}$
- Total number of Atoms $=$ mole $(\mathrm{n}) \times \mathrm{N}_{\mathrm{A}} \times$ No. of atoms present in one molecule (atomicity).
- Total number of Electrons $=$ mole $(\mathrm{n}) \times \mathrm{N}_{\mathrm{A}} \times$ No. of electrons present in one atom.
- The total charge on any ion = mole (n) x NA x charge on one ion $\times 1.6 \times 10^{-19} \mathrm{C}$
Also read -
Recommended topic video on (Mole Concept Basic )
Some Solved Examples
Que 1: Number of atoms in 558.5 gram Fe (at. wt. of Fe = 55.85 g mol-1) is:
1) twice that in 60 g carbon
2)$6.023 \times 10^{22}$
3) half that in 8 g He
4) 558.5 × 6.023 × 1023
Solution
Number of moles of Fe atom$=558.5 / 55.85=10 \mathrm{Mole}$
Total number of atom
$=10 \times 6.022 \times 10^{23}=6.022 \times 10^{24}=10 \times \mathrm{NA}$
12 g of carbon means Avogadro's number,
therefore, 60 g of carbon means, 5 moles of carbon and thus contains 5×NA atoms, twice of this equals 10NA atoms of carbon.
Hence, the answer is the option (1).
Que 2: If we consider that 1/6, in place of 1/12, the mass of the carbon atom is taken to be the relative atomic mass unit, the mass of one mole of a substance will
1) decrease twice
2) increase two-fold
3) remain unchanged
4) be a function of the molecular mass of the substance.
Solution
When you consider 1/6 in place of 1/12 since the mass of the carbon atom is still the same, new amu1 = 2amu ( where amu1 is the new amu unit and amu is the old amu unit). So now Na1 (Avogadro's number) becomes half ( Na/2) since the weight of carbon corresponding to atomic weight becomes 6gm in place of 12. ( where Na1 is the new Avogadro's number and Na is the old Avogadro's number). And we know that the mass of one mole of a substance is = Na x amu. So new atomic mass = Na1 $\times$ amu1 $=$ Na/2 $\times$ 2amu $=$ Na $\times$ amu $=$ old atomic weight.
Hence, the answer is the option (3).
Que 3: What is the total number of moles if we have a mixture of 34g of Ammonia, 78g of Benzene, and 10g of Hydrogen?
1) 8
2) 9
3) 7
4) 10
Solution
Number of moles of ammonia = mass of ammonia molar mass of ammonia =34/17=2
Number of moles of benzene =78/78=1
Number of moles of hydrogen =10/2=5
Total number of moles = 2 + 1 + 5 = 8
Hence, the answer is (8).
Que 4: We have a substance x. What is the molar mass (in g) of x if 2 moles of it weigh 196g?
1) 147
2) 196
3) 98
4) 49
Solution
Molar mass of x=196/2g=98g
Hence, the answer is (98g).
Que 5. 14 g of Nitrogen gas and 22 g of CO2 gas are mixed together. Find the volume of the gaseous mixture at STP.
(Response should be up to only one decimal digit like 78.7).
1) 22.4
2) 11.2
3) 44.8
4) 52.8
Solution
Number of Moles of a gas at STP -
no. of moles of a gas at STP = given volume of gas / 22.4 liter
Moles of N2=0.5
moles of CO2=0.5
So total moles 0.5+0.5=1
So vol. at STP =1×22.4=22.4lit
Hence, the answer is the option (1).
Practice more questions from the link given below
For more questions to practice, the following MCQs will help in preparation of competitive examinations
Conclusion
The number $6.022 \times 10^{23}$ is called the Avogadro number or Avogadro constant. It is denoted by NA in honor of nineteenth-century Italian scientist, Amedeo Avogadro. In other words, a mole is an Avogadro number of particles. The mass of one mole of any substance will be its molar mass and it contains $6.022 \times 10^{23}$ particles of that substance.
Also, check
Frequently Asked Questions (FAQs)
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol).
For elements: It's the atomic mass in grams.
For compounds: Sum of the constituent atomic masses (e.g., water = ~18.015 g/mol).
The value of one mole is 6.022 × 1023
The relationship is defined by the formula: concentration (C) = moles of solute (n) / volume of solution (V), where concentration is typically expressed in moles per liter (M). This formula allows for the determination of how much solute is present in a given volume of solution.
Avogadro's law states that equal volumes of gases, at the same temperature and pressure, contain an equal number of moles. This principle supports the mole concept, demonstrating how the mole measures the number of atoms in a given volume of gas.
The number of atoms in a mixture can be calculated by first determining the number of moles of each component in the mixture using the molar masses of the components. The number of atoms of each element in the mixture can then be calculated by multiplying the number of moles of each component by the subscript of that element in the chemical formula for that component. The total number of atoms of each element in the mixture can then be calculated by adding the number of atoms from each component.
18 grams of water means the same as 1 mole of water, and in this Avogadro number of water molecules are present, which means 6.02×1023 molecules.