Electronic Configuration of First 30 Elements - Meaning, Definition, Elements, Functions, FAQs
Have you ever thought about why sodium is highly reactive while neon is completely inert? Or why do elements in the same group show similar chemical properties? The answer lies in the electronic configuration, the systematic distribution of electrons into different shells, subshells, and orbitals. Electronic configuration is an organized alignment of electrons in atomic orbitals according to the energy levels. This arrangement of electrons is directed by concepts like Hund's rule, Pauli exclusion, and the Aufbau rule.
This Story also Contains
- Let's Understand Electronic Configuration
- How To Write The Electronic Configuration Of Elements
- Rules for Writing Electronic Configurations Of Elements
- List Of First 30 Elements: Atomic Number, Element, And Electronic Configuration
- What Function Does the Electronic Configuration Of Elements Play?
- Some Solved Examples

Electronic configuration is helpful in determining the chemical properties, reactivity, and position in the periodic table. It also influences what type of bond the elements will form, the valency, and the ability to donate or accept electrons during a chemical reaction. The trend in the periodic table, like ionization enthalpy, electronegativity, and atomic size, can also be explained through this arrangement of electrons. The following electronic configurations of the first 30 elements are mentioned, which will help in understanding the trends they obey.
Let's Understand Electronic Configuration
An electronic Configuration, also known as an electronic structure, is the arrangement of electrons at different energy levels surrounding an atomic nucleus. The electronic configuration of a molecule is the distribution of electrons in distinct molecular orbitals. The importance of the molecule cannot be overstated. It is possible to determine the number of electrons in bonding and antibonding molecular orbitals from a molecule's or molecular ion's electronic configuration.
-
The electrical configuration of an element is used to figure out where electrons are located in that element.
-
From the lowest to the highest energy level, electrons are arranged in ascending order.
-
The electrical configuration of an element is largely determined by its atomic number.
-
The electrical configuration of an atom is helpful in determining an element's valency, which aids in determining the element's reactivity.
-
The atomic spectra can also be interpreted using the electronic configuration.
-
Noble gases with totally filled outermost electrons, such as Neon, Argon, and Helium, are the most stable. Noble gases have filled valence shells, which give them their inertness.
-
Copper and chromium have a peculiar electrical structure in which the 3d- orbitals are filled first, rather than the 4s orbitals.
-
In chromium([Ar] 3d5 4s1) the d-orbital, which is filled with single electrons, boosts the atom's stability. Similarly, the d-orbital of Copper [Ar] 3d10 4s1 is totally filled with paired electrons, ensuring the atomic structure's stability.
How To Write The Electronic Configuration Of Elements
The rules mentioned below are to be followed while writing electronic configurations. To get different quantum numbers, we first have to extract various information, such as the number of electrons, possible number of various shells and orbitals, energy levels, etc., of elements by using the periodic table.
The electronic configuration is as follows: $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^1$. Its 19 electrons can be divided into shells in the following ways:
K shell (n=1) equals 2, L shell (n=2) equals 8, M shell (n=3) equals 8, and N shell (n=4) equals 1.
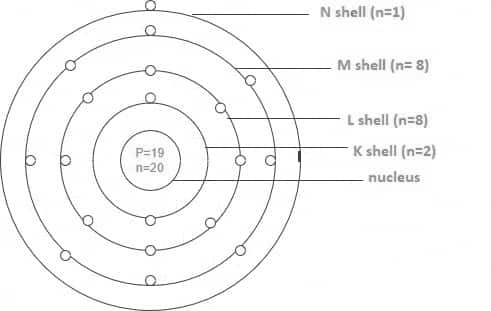
Let us take an example of the electronic configuration of iron, which is mentioned below, for a better understanding of the topic
Significance of the electronic configuration of Iron
Iron is a one-of-a-kind element that exists both outside and inside us. The atomic number of Iron is 26.Iron has 8 valence electrons and an electron configuration of 1s2 2s1 2p6 3s2 3p6 3d6 4s2, which means it has
-
K shell – 2 electrons,
-
L shell – 8 electrons,
-
M shell – 14 electrons, and
- N shell – 2 electrons.
Various properties of Iron can be explained using the electronic configuration. Like, Iron is a silvery white metal that is ductile and malleable under normal conditions. Iron is a medium-activity metal that extracts hydrogen from water solutions of strong acids like HCl and sulfuric acid, resulting in the formation of iron salts. These can be explained by the number of valence shell electrons in Iron.
Rules for Writing Electronic Configurations Of Elements
-
The Aufbau principle states that before occupying an orbital associated with a higher energy level, electrons must entirely fill the atomic orbitals of the previous energy level. In the sequence of increasing orbital energy levels, electrons occupy orbitals of lower energy first.
-
According to Pauli's exclusion principle, no two electrons may have the same values for all four quantum numbers. As a result, each subshell of an orbital may only hold a maximum of two electrons, both of which must have opposite spins for the spin quantum number to be different.
-
Hund's rule of maximum multiplicity states that all subshells in an orbital must be occupied singly before any subshell can be twice occupied. Furthermore, all electrons in singly occupied subshells must have the same spin (in order to maximize the overall spin).
List Of First 30 Elements: Atomic Number, Element, And Electronic Configuration
|
ATOMIC NUMBER 1 to 30 |
ELEMENT |
ELECTRONIC CONFIGURATION |
|
1 |
HYDROGEN (H) | $1 \mathrm{~s}^1$ |
|
2 |
HELIUM (He) | $1 \mathrm{~s}^2$ |
|
3 |
LITHIUM (Li) | $1 s^2 2 s^1$ |
|
4 |
BERYLLIUM (Be) | $1 s^2 2 s^2$ |
|
5 |
BORON (B) | $1 s^2 2 s^1 2 p^1$ |
|
6 |
CARBON (C) | $1 s^2 2 s^1 2 p^2$ |
|
7 |
NITROGEN (N) | $1 s^2 2 s^1 2 p^3$ |
|
8 |
OXYGEN (O) | $1 s^2 2 s^1 2 p^4$ |
|
9 |
FLUORINE (F) | $1 s^2 2 s^1 2 p^5$ |
|
10 |
NEON (Ne) | $1 s^2 2 s^1 2 p^6$ |
|
11 |
SODIUM (Na) | $1 s^2 2 s^1 2 p^6 3 s^1$ |
|
12 |
MAGNESIUM (Mg) | $1 s^2 2 s^1 2 p^6 3 s^2$ |
|
13 |
ALUMINIUM (Al) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^1$ |
|
14 |
SILICON (Si) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^2$ |
|
15 |
PHOSPHORUS (P) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^3$ |
|
16 |
SULPHUR (S) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^4$ |
|
17 |
CHLORINE (Cl) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^5$ |
|
18 |
ARGON (Ar) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6$ |
|
19 |
POTASSIUM (K) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 4 s^1$ |
|
20 |
CALCIUM (Ca) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 4 s^2$ |
|
21 |
SCANDIUM (Sc) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^1 4 s^2$ |
|
22 |
TITANIUM (Ti) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^2 4 s^2$ |
|
23 |
VANADIUM (V) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^3 4 s^2$ |
|
24 |
CHROMIUM (Cr) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^5 4 s^1$ |
|
25 |
MANGANESE (Mn) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^5 4 s^2$ |
|
26 |
IRON (Fe) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^6 4 s^2$ |
|
27 |
COBALT (Co) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^7 4 s^2$ |
|
28 |
NICKEL (Ni) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^8 4 s^2$ |
|
29 |
COPPER (Cu) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^{10} 4 s^1$ |
|
30 |
ZINC (Zn) | $1 s^2 2 s^1 2 p^6 3 s^2 3 p^6 3 d^{10} 4 s^2$ |
The above table contains atomic numbers 1 to 30 elements with symbols and electronic configuration. The electronic configuration of elements can also be written in the form of the electronic configuration of nearest noble gases.
For example- In this table, we can represent the electronic configuration of elements from 21 to 30 in the form of Ar as Argon (atomic number - 18) is the nearest noble gas for the first series elements of the d-block.
What Function Does the Electronic Configuration Of Elements Play?
The chemical characteristics of elements are largely determined by their electronic arrangement. Despite their small size, electrons are responsible for determining the nature of the elements. They determine the valency, ionization potential, ionization enthalpy, chemical bonding, and practically all other chemical properties of the element. When an element lacks an electron, it is classified as an electron acceptor, and when it has an excess electron, it is classified as an electron giver. As a result, electrical configuration, like the electron, is a deciding factor.
Also Read:
| Electronic Configuration of Iron |
| Homologous Series |
| Modern Periodic Table Modern Periodic Law |
| Periodic Trends in Ionisation Enthalpy of Elements |
Recommended topic video on (Electronic Configuration of First 30 Elements)
Some Solved Examples
Example 1. Which law indicates the pairing of electrons in the same orbital?
1) Newton’s first law
2) (correct) Hund’s rule
3) Aufbau principle
4) Pauli exclusion principle
Solution
Hund’s rule states that “pairing of electrons in the orbitals belonging to the same subshell (p, d or f) does not take place until each orbital belonging to that subshell has got one electron each. It is singly occupied”.
Hence, the answer is option (2).
Example 2. The number of electrons that Vanadium (Z = 23) has in p-orbitals is equal to ______
1) (correct) 12
2) 11
3) 10
4) 9
Solution
The electronic configuration of V(Z= 23) is given as
1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3
Thus, there are 12 electrons in the p-orbitals of Vanadium.
Hence, the answer is option (1).
Example 3. Identify the element for which the electronic configuration in +3 oxidation state is [ Ar ] 3d5:
1) Ru
2) Mn
3) Co
4) Fe
Solution
As we have learned,
Fe has an electronic configuration of [ Ar ] 4s23d6
So, Fe 3+ has an electronic configuration [ Ar ] 3d5.
Hence, the answer is option (4).
Example 4. Which of the following configurations represents a noble gas?
A. $1 s^2 2 s^2 2 p^6$
B. $1 s^2 2 s^2 2 p^6 3 s^2 3 p^5$
C. $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6$
D. $1 s^2 2 s^2 2 p^3$
Solution- $1 s^2 2 s^2 2 p^6 3 s^2 3 p^6$ totals 18 electrons = Argon (Ar), a noble gas.
Noble gases have full outer shells, making them stable.
Practice More Questions With The Link Given Below
| Introduction of Periodic Table practice question and MCQs |
| Electronic Configuration in Periods and Groups practice question and MCQs |
Also read -
Frequently Asked Questions (FAQs)
Electron correlation refers to the interaction between electrons in an atom. It's not fully accounted for in simple electronic configuration models but is important for understanding the detailed behavior of multi-electron atoms.
Iron's electronic configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶) includes unpaired electrons in the 3d orbitals. These unpaired electrons with parallel spins give iron its ferromagnetic properties.
Zinc (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰) has a fully filled 3d sublevel, unlike most transition metals. This gives zinc properties more similar to main group elements than typical transition metals.
Sulfur's electronic configuration (1s² 2s² 2p⁶ 3s² 3p⁴) allows it to use its empty 3d orbitals to accommodate more than 8 electrons in its valence shell, forming expanded octets in some compounds.
The diagonal rule states that elements diagonally related in the periodic table often have similar properties. This is because they have similar ratios of valence electrons to electron shells, despite different total electron counts.
Chromium's electronic configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵) shows one 4s electron moving to the 3d orbital, resulting in a half-filled 4s and half-filled 3d sublevel, which is more stable than the expected [Ar] 4s² 3d⁴.
Nitrogen's electronic configuration (1s² 2s² 2p³) has three unpaired electrons in its 2p orbitals. These three electrons can form three covalent bonds with another nitrogen atom, resulting in the very stable triple bond in N₂.
Diamagnetic substances have all paired electrons in their electronic configuration, while paramagnetic substances have one or more unpaired electrons. This affects how the substance interacts with magnetic fields.
Aluminum's electronic configuration (1s² 2s² 2p⁶ 3s² 3p¹) has three valence electrons. It can lose all three to form ionic bonds (Al³⁺) or share them to form covalent bonds, giving it versatile bonding capabilities.
Copper's electronic configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰) deviates from the expected [Ar] 4s² 3d⁹. One 4s electron is moved to the 3d orbital, resulting in a fully filled 3d sublevel, which is more stable.