First 20 Elements of the Periodic Table: Names, Symbols & Atomic Numbers
Have you ever wondered why hydrogen is so light, yet calcium helps make our bones strong? Why does helium fill balloons, while sodium reacts violently with water? We will get the answers to all these questions after studying the first 20 elements of the periodic table. The first 20 elements of the Periodic table form the core of Chemistry. Every element in the periodic table is arranged based on its chemical and physical properties, such as atomic number, electronic configuration, and recurring chemical properties. These are the very basic elements, but they form the basis for the entire chemistry.
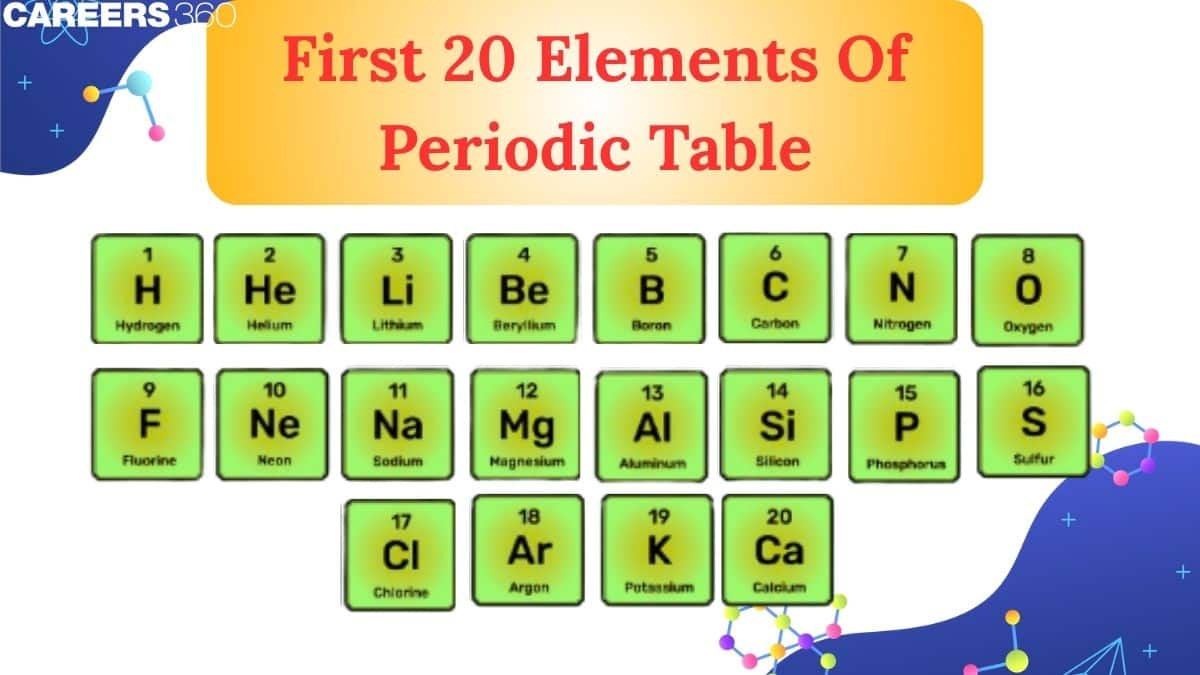
These elements represent the wide range of Metals, Non-metals, and Noble Gases. From the importance of Carbon in Organic life to the essential role of Calcium in bones and teeth, these elements play an important role in everyday life. Inside the periodic table, elements are arranged in the form of rows and columns. The rows are called periods, and the columns are called groups. The basic understanding of these elements helps to build a strong foundation in Chemistry.
Atomic Numbers And Symbols of The First 20 Elements
|
Atomic numbers |
Element |
Symbol |
|
|
Hydrogen |
H |
|
|
Helium |
He |
|
|
Lithium |
Li |
|
|
Berilliyum |
Be |
|
|
Boron |
B |
|
|
Carbon |
C |
|
|
Nitrogen |
N |
|
|
Oxygen |
O |
|
|
Fluorine |
F |
|
|
Neon |
Ne |
|
|
Sodium |
Na |
|
|
Magnesium |
Mg |
|
|
Aluminium |
Al |
|
|
Silicon |
Si |
|
|
Phosphorous |
P |
|
|
Sulfur |
S |
|
|
Chlorine |
Cl |
|
|
Argon |
Ar |
|
|
Potassium |
K |
|
|
calcium |
Ca |
An in-depth look at the first 20 elements in the periodic table is useful. Six of these elements make up nearly all of the mass of the human body. The first 20 elements provide an excellent overview of the various element groups. They can also be found in more common chemical processes. The elements are given in ascending atomic numbers from 1 to 20 order. The atomic number refers to the number of protons in each element's atoms.
First 20 Elements of The Periodic Table
The periodic table is the representation of elements in increasing order of their atomic number.
Name And Symbols Of Elements
The atomic number, element name, or element symbol can all be used to identify elements. A one- or two-letter abbreviation of the name serves as the emblem. Some symbols, on the other hand, allude to old element names. The symbol for sodium, for given as Na. This is a reference to the Latin word natrium, which was once used to refer to caustic soda. In Latin, the name of the atomic no of sodium is found to be atrium. Potassium's symbol is K, which is derived from the Latin word kalium, which denoted alkali or potash. An element symbol's initial letter is capitalised. It's lowercase when there's a second letter.
Examination Ff 1 To 20 Elements Of The Periodic Table
1. Hydrogen
Under normal circumstances, hydrogen is a nonmetallic, colourless gas. It transforms into an alkali metal under great pressure. This element has three isotopes, each with a different amount of neutrons in its atoms. Protium is the most prevalent isotope. Deuterium and tritium are the other two.
-
The atomic number of Hydrogen is 1
-
H is its symbol
-
It is estimated to have an atomic mass value of 1.008 amu
-
H is assigned 1s1 configuration
-
It is a Nonmetal belonging to group 1 and s-block
2. Helium
-
Helium is a light gas with no visible colour.
-
The atomic number of Helium is 2
-
He is its symbol
-
It is estimated to have an atomic mass value of 4.002 amu
-
He is assigned 1s2 configuration
-
It belongs to group 18 and s-block
3. Lithium
It is a highly reactive solid metal with a silver colour
-
The atomic number of Lithium is 3
-
Li is its symbol
-
It is estimated to have an atomic mass value of 6.94 amu
-
Li is assigned [He] 2s1 configuration
-
It is an alkali metal belonging to group 1 and s-block.
4. Beryllium
It is a solid material with a shiny grey- white appearance
-
The atomic number of beryllium is 4
-
Be is its symbol
-
It is estimated to have an atomic mass value of 9.012 amu
-
Be is assigned [He] 2s2 configuration
-
It is an alkaline earth metal belonging to group 2 and s-block.
5. Boron
-
The atomic number of Boron is 5
-
B is its symbol
-
It is estimated to have an atomic mass value of 10.81 amu
-
B is assigned [He] 2s2 2p1 configuration
-
It is a metalloid belonging to group 13 and p-block.
6. Carbon
-
The atomic number of carbon is 6
-
C is its symbol
-
It is estimated to have an atomic mass value of 12.011 amu
-
C is assigned [He] 2s2 2p2 configuration
-
It is a metalloid belonging to group 14 and p-block
7. Nitrogen
-
The atomic number of nitrogen is 7
-
N is its symbol
-
It is estimated to have an atomic mass value of 14.007 amu
-
N is assigned [He] 2s2 2p3 configuration
-
It belongs to group 15 and the p-block.
8. Oxygen
-
The atomic number of oxygen is 8
-
O is its symbol
-
It is estimated to have an atomic mass value of 16 amu
-
O is assigned [He] 2s2 2p4 configuration
-
It belongs to group 16 and p-block
9. Fluorine
-
The atomic number of fluorine is 9
-
F is its symbol
-
It is estimated to have an atomic mass value of 18.989 amu
-
F is assigned [He] 2s2 2p5 configuration
-
It belongs to group 17 and p-block
10. Neon
-
The atomic number of neon is 10
-
Ne is its symbol
-
It is estimated to have an atomic mass value of 20.179 amu
-
N is assigned [He] 2s2 2p6 configuration
-
It belongs to group 18 and the p-block
11. Sodium
-
The atomic number of sodium is 11
-
Na is the symbol of sodium
-
The Latin name of sodium is Natrium
-
It is estimated to have an atomic mass value of 22.989 amu
-
F is assigned [Ne] 3s1 configuration
-
It belongs to group 1 and the s-block
12. Magnesium
-
The atomic number of magnesium is 12
-
Mg is its symbol
-
It is estimated to have an atomic mass value of 24.309 amu
-
Mg is assigned [Ne] 3s2 configuration
-
It belongs to group 2 and the s-block
13. Aluminium
-
The atomic number of aluminium is 13
-
Al is its symbol
-
It is estimated to have an atomic mass value of 26.968 amu
-
Al is assigned [Ne] 3s2 3p1 configuration
-
It belongs to group 13 and the p-block
14. Silicon
-
The atomic number of Silicon is 14
-
Si is its symbol
-
It is estimated to have an atomic mass value of 28.085 amu
-
Si is assigned [Ne] 3s2 3p2 configuration
-
It belongs to group 14 and the p-block.
15. Phosphorus
-
The atomic number of Phosphorus is 15
-
P is its symbol
-
It is estimated to have an atomic mass value of 30.9737 amu
-
P is assigned [Ne] 3s2 3p3 configuration
-
It belongs to group 15 and the p-block
16. Sulphur
-
The atomic number of Sulfer is 16
-
S is its symbol
-
It is estimated to have an atomic mass value of 32.09 amu
-
S is assigned [Ne] 3s2 3p4 configuration
-
It belongs to group 16 and the p-block
17. Chlorine
-
The atomic number of Chlorine is 17.
-
Cl is its symbol
-
It is estimated to have an atomic mass value of 35.45 amu
-
Cl is assigned [Ne] 3s2 3p5 configuration
-
It belongs to group 17 and the p-block
18. Argon
-
The atomic number of argon is 18
-
Ar is its symbol
-
It is estimated to have an atomic mass value of 39.948 amu
-
Ar is assigned [Ne] 3s2 3p6 configuration
-
It belongs to group 18 and the p-block
19. Potassium
-
The atomic number of Potassium is 19
-
The Latin name of potassium is found to be Kalium
-
K is its symbol
-
It is estimated to have an atomic mass value of 39.093 amu
-
K is assigned [Ar] 4s1 configuration
-
It belongs to group 1 and the s-block
20. Calcium
-
The atomic number of Calcium is 20.
-
Ca is its symbol
-
It is estimated to have an atomic mass value of 40.10 amu
-
Ca is assigned [Ar] 4s2 configuration
-
It belongs to group 14 and the s-block
Also Read:
Recommended topic video
Some Solved Examples
Example.1: Choose the correct option:
1) (correct)The period of the element is determined by its highest shell
2)The period of the element is determined by its last orbital
3)The period of the element is determined by its valence shell electrons
4)The period of the element is determined by its valency
Solution
The period of the element is determined by its highest shell.
Hence, the answer is option (1).
Example.2 Which pair of atomic numbers represents s-block elements
1)7,15
2)6,12
3)9,17
4) (correct)3,12
Solution
Out of the given elements, Z=3 (Li) and Z= 12 (Mg) belong to the s Block of the periodic table.
Hence, the answer is option (4).
Example 3. Newland’s octave law was successful in arranging:
1)Heavier elements
2) (correct)Lighter elements
3)Both
4)None
Solution
Newland’s octave law was successful in arranging lighter elements. After calcium, this law did not work accordingly.
Hence, the answer is option (2).
Practice More Questions From The link Given below
For more questions to practice, the following MCQs will help in the preparation for competitive examinations
Summary
The first 20 elements of the periodic table possess a range of chemical properties and their uses from simplest to most abundant elements like hydrogen and some essential elements of earth such as carbon, nitrogen and hydrogen these three elements are the main building blocks of many processes and also plays a vital role in the chemistry field and has various technological applications. Their characteristics are very diverse and interconnected. The first 20 elements of the periodic table contain metals such as sodium, magnesium, and aluminium. Metalliods such as silicon, and boron. Also, contains noble gases like neon and argon. Understanding all these elements helps us to appreciate the balance of nature and their application in chemistry.
Also read -
Frequently Asked Questions (FAQs)
Beryllium shows some properties similar to aluminum due to their diagonal relationship in the periodic table. Both have a charge-to-size ratio that leads to similar chemical behaviors. Beryllium, like aluminum, forms amphoteric oxides and hydroxides, meaning they can react as both acids and bases. This similarity arises because beryllium's small size and high charge density make its chemistry more similar to aluminum than to its group members like magnesium. This diagonal relationship is an important concept in understanding periodic trends beyond simple group and period patterns.
Ionization energy explains the formation of different oxidation states in transition metals like scandium and titanium. These elements can lose multiple electrons because the energy required to remove subsequent electrons (2nd, 3rd ionization energies) is not significantly higher than the first. This is due to the similar energies of their 4s and 3d orbitals. For example, titanium can form Ti²⁺, Ti³⁺, and Ti⁴⁺ ions because the energy required to remove these electrons is chemically accessible under various conditions, allowing for multiple oxidation states.
The shielding effect explains potassium's larger atomic size compared to sodium. Potassium's outer electron is in the 4s orbital, while sodium's is in the 3s orbital. The additional inner electron shell in potassium (the 3p electrons) shields the outer electron from the nuclear charge more effectively than in sodium. This increased shielding reduces the effective nuclear charge experienced by the outer electron, allowing it to exist further from the nucleus, resulting in a larger atomic radius for potassium.
Magnesium forms a stronger ionic bond with oxygen than with fluorine, despite fluorine's higher electronegativity, due to the charge difference in the resulting ions. When magnesium bonds with oxygen, it forms Mg²⁺ and O²⁻ ions, resulting in a 2:2 charge ratio. With fluorine, it forms Mg²⁺ and F⁻ ions, a 2:1 charge ratio. The higher charges in the Mg-O bond lead to stronger electrostatic attraction. Additionally, the smaller size of the O²⁻ ion compared to two F⁻ ions allows for closer packing and stronger ionic interactions in magnesium oxide.
While electron affinity and electronegativity both generally increase from left to right across a period, they differ in their group trends. Electronegativity typically decreases down a group, but electron affinity doesn't follow a consistent trend down groups. For instance, chlorine has a higher electron affinity than fluorine, despite fluorine being more electronegative. This difference arises because electron affinity is more influenced by the specific electronic structure of each element, while electronegativity is more directly related to nuclear charge and atomic size.
Phosphorus has multiple allotropes while nitrogen typically exists as diatomic molecules due to differences in their atomic size and bonding capabilities. Nitrogen, being smaller, forms very strong triple bonds in N₂ molecules, making this form highly stable. Phosphorus, being larger, can form single, double, or triple bonds with itself. This versatility allows phosphorus to create various structural arrangements (allotropes) like white, red, and black phosphorus, each with different properties and stabilities.
The concept of effective nuclear charge explains why aluminum has a smaller atomic radius than sodium. Although aluminum has more electrons, its outer electrons are in the same shell (n=3) as sodium's. However, aluminum has a higher nuclear charge (13 protons vs. 11 for sodium) and less effective shielding from its inner electrons. This results in a greater effective nuclear charge in aluminum, pulling its outer electrons closer to the nucleus and creating a smaller atomic radius despite having more electrons overall.
Argon has a higher boiling point than neon, despite both being noble gases, due to stronger intermolecular forces. Although both are nonpolar, argon has more electrons and thus a larger electron cloud. This larger electron cloud is more easily polarized, leading to stronger instantaneous dipole-induced dipole forces (London dispersion forces) between argon atoms. These stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point for argon compared to neon.
Electronegativity, the ability of an atom to attract shared electrons in a bond, explains bond polarity trends across the first 20 elements. Electronegativity generally increases from left to right across a period and decreases down a group. This means that bonds between elements on the left (like sodium) and right (like chlorine) of the periodic table tend to be more polar. The greater the electronegativity difference, the more polar the bond. This concept helps predict the nature of chemical bonds and the properties of compounds formed by these elements.
The melting point trend across period 3 shows an irregular pattern due to changes in bonding types and strengths. Sodium and magnesium have metallic bonds, with magnesium's higher melting point due to stronger bonding. Aluminum continues this trend. However, silicon has strong covalent network bonding, causing a sharp increase in melting point. Phosphorus, sulfur, and chlorine form molecular structures with weaker intermolecular forces, resulting in lower melting points. This variation in bonding types causes the irregular melting point trend.