118 Elements and Their Symbols and Atomic Numbers
Have you wondered why hydrogen has the symbol H while potassium is represented by K? What is the logic behind assigning each element a unique atomic number? Well, you can find the answer to these questions by reading this article 118 Elements and Their Symbols and Atomic Numbers. In the periodic table, there are 118 elements, each of them has a unique name, symbol, and atomic number. Arrangements of elements in the periodic table depend on their atomic number and properties. These elements represent the wide range of Metals, Non-metals, and Noble Gases.
This Story also Contains
- Atomic Number
- What Does The Symbol Of An Element Mean?
- List Of 118 Elements: Name, Symbol, And Atomic Number
- 118 Elements In The Periodic Table
- Some Solved Examples
The first element, or the element whose atomic number is 1, is Hydrogen, represented by ‘H’, and the last element, whose atomic number is 118, is Oganesson, represented by the symbol ‘Og’. Most of the symbols are similar to the names of the elements, but there are certain symbols of objects with Latin roots. Its example is the lead described by Pb, which is derived from its Latin name "Plumbum". One such example would be the 'Fe' symbol used to represent the metal and could be associated with the Latin word iron, "Ferrum". Scroll down to know more about 118 Elements and Their Symbols and Atomic Numbers
Atomic Number
118 elements and their symbols and atomic numbers are arranged from left to right and top to bottom in order of increasing atomic number. In the modern periodic table, the elements' names and symbols are listed in order of increasing atomic number. Order generally coincides with increasing atomic mass. The rows are called periods. In a periodic table arranged in order of increasing atomic number, elements and symbols having similar chemical properties naturally line up in the same group.
The current table, today, contains a total of as element 118 elements and their symbols and atomic numbers. Since the names of the elements can be long and complex to use, they are indicated by using a symbol. Each item has a unique symbol.
|
Related Topics: |
What Does The Symbol Of An Element Mean?
A sign or symbol is a note usually consisting of one or two letters that are used to represent a chemical object. There are also 3-character symbols. These are things that have just been merged and so-called for a while. There are some rules for marking. The first letter of the mark is usually capitalized, while the second (or third) letter is usually written in small letters. Eg. - Ca for Calcium, He for Helium, etc. If an item mark contains only one letter, it is always capitalized.
Eg. -Nitrogen (N), Oxygen (O), etc.
Symbols are often used to represent objects in a timeline. The chemical formula and proportions must also be used for those properties.
The following table provides a list of 118 elements and their symbols, atomic numbers, and the number of atoms.
Periodic table of elements with names and their symbols(List of elements)
List Of 118 Elements: Name, Symbol, And Atomic Number
The following table provides a list of 118 elements and their symbols, atomic numbers, and the number of atoms.
|
Name of the Element |
Symbol of the Element |
Atomic Number |
|
Hydrogen |
H |
1 |
|
Helium |
He |
2 |
|
Lithium |
Li |
3 |
|
Beryllium |
Be |
4 |
|
Boron |
B |
5 |
|
Carbon |
C |
6 |
|
Nitrogen |
N |
7 |
|
Oxygen |
O |
8 |
|
Fluorine |
F |
9 |
|
Neon |
Ne |
10 |
|
Sodium |
Na |
11 |
|
Magnesium |
Mg |
12 |
|
Aluminium |
Al |
13 |
|
Silicon |
Si |
14 |
|
Phosphorus |
P |
15 |
|
Sulfur |
S |
16 |
|
Chlorine |
Cl |
17 |
|
Argon |
Ar |
18 |
|
Potassium |
K |
19 |
|
Calcium |
Ca |
20 |
|
Scandium |
Sc |
21 |
|
Titanium |
Ti |
22 |
|
Vanadium |
V |
23 |
|
Chromium |
Cr |
24 |
|
Manganese |
Mn |
25 |
|
Iron |
Fe |
26 |
|
Cobalt |
Co |
27 |
|
Nickel |
Ni |
28 |
|
Copper |
Cu |
29 |
|
Zinc |
Zn |
30 |
|
Gallium |
Ga |
31 |
|
Germanium |
Ge |
32 |
|
Arsenic |
As |
33 |
|
Selenium |
Se |
34 |
|
Bromine |
Br |
35 |
|
Krypton |
Kr |
36 |
|
Rubidium |
Rb |
37 |
|
Strontium |
Sr |
38 |
|
Yttrium |
Y |
39 |
|
Zirconium |
Zr |
40 |
|
Niobium |
Nb |
41 |
|
Molybdenum |
Mo |
42 |
|
Technetium |
Tc |
43 |
|
Ruthenium |
Ru |
44 |
|
Rhodium |
Rh |
45 |
|
Palladium |
Pd |
46 |
|
Silver |
Ag |
47 |
|
Cadmium |
Cd |
48 |
|
Indium |
In |
49 |
|
Tin |
Sn |
50 |
|
Antimony |
Sb |
51 |
|
Tellurium |
Te |
52 |
|
Iodine |
I |
53 |
|
Xenon |
Xe |
54 |
|
Cesium |
Cs |
55 |
|
Barium |
Ba |
56 |
|
Lanthanum |
La |
57 |
|
Cerium |
Ce |
58 |
|
Praseodymium |
Pr |
59 |
|
Neodymium |
Nd |
60 |
|
Promethium |
Pm |
61 |
|
Samarium |
Sm |
62 |
|
Europium |
Eu |
63 |
|
Gadolinium |
Gd |
64 |
|
Terbium |
Tb |
65 |
|
Dysprosium |
Dy |
66 |
|
Holmium |
Ho |
67 |
|
Erbium |
Er |
68 |
|
Thulium |
Tm |
69 |
|
Ytterbium |
Yb |
70 |
|
Lutetium |
Lu |
71 |
|
Hafnium |
Hf |
72 |
|
Tantalum |
Ta |
73 |
|
Tungsten |
W |
74 |
|
Rhenium |
Re |
75 |
|
Osmium |
Os |
76 |
|
Iridium |
Ir |
77 |
|
Platinum |
Pt |
78 |
|
Gold |
Au |
79 |
|
Mercury |
Hg |
80 |
|
Thallium |
Tl |
81 |
|
Lead |
Pb |
82 |
|
Bismuth |
Bi |
83 |
|
Polonium |
Po |
84 |
|
Astatine |
At |
85 |
|
Radon |
Rn |
86 |
|
Francium |
Fr |
87 |
|
Radium |
Ra |
88 |
|
Actinium |
Ac |
89 |
|
Thorium |
Th |
90 |
|
Protactinium |
Pa |
91 |
|
Uranium |
U |
92 |
|
Neptunium |
Np |
93 |
|
Plutonium |
Pu |
94 |
|
Americium |
Am |
95 |
|
Curium |
Cm |
96 |
|
Berkelium |
Bk |
97 |
|
Californium |
Cf |
98 |
|
Einsteinium |
Es |
99 |
|
Fermium |
Fm |
100 |
|
Mendelevium |
Md |
101 |
|
Nobelium |
No |
102 |
|
Lawrencium |
Lr |
103 |
|
Rutherfordium |
Rf |
104 |
|
Dubnium |
Db |
105 |
|
Seaborgium |
Sg |
106 |
|
Bohrium |
Bh |
107 |
|
Hassium |
Hs |
108 |
|
Meitnerium |
Mt |
109 |
|
Darmstadtium |
Ds |
110 |
|
Roentgenium |
Rg |
111 |
|
Copernicium |
Cn |
112 |
|
Nihonium |
Nh |
113 |
|
Flerovium |
Fl |
114 |
|
Moscovium |
Mc |
115 |
|
Livermorium |
Lv |
116 |
|
Tennessine |
Ts |
117 |
|
Oganesson |
Og |
118 |
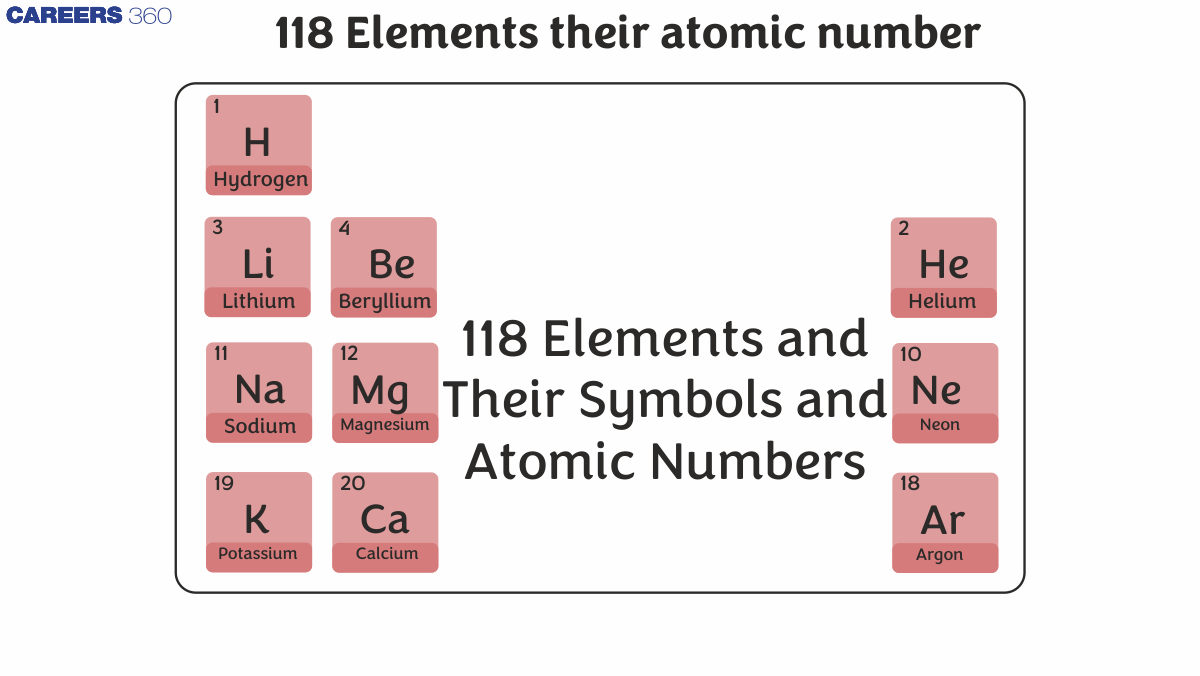
118 Elements In The Periodic Table
118 Elements and Their Symbols and Atomic Numbers are represented in the periodic table in the following manner:
.png)
Also Read,
Some Solved Examples
Example.1 What is the symbol of silver?
1) Si
2) Ar
3) (correct)Ag
4) Sb
Solution
The symbol of silver is Ag derived from the Latin word Argentum.
Hence, the answer is the option (4).
Example.2 Which of the following represents elements in increasing order of their atomic size?
1) I, Br, Cl
2) N, O, F
3) (correct)Be, Mg, Ca
4) Se, S, O
Solution
As we have learned
Vander Waals Radius - It represents the overall size of the atom which includes its valence shell in a non-bonded situation. It is half the distance between two similar atoms in separate molecules in a solid.Vanderwaals radius > metallic radius > covalent radius. Atomic size increases as we move down in a group
Hence, the answer is the option (3).
Example.3 Which of the following has the largest size
1) (correct)Al
2) Al+
3) Al+2
4) Al+3
Solution
With successive ionisations, the size of the species decreases. This is due to an increase in the effective nuclear charge which tends to hold the valence electrons more tightly thus leading to a reduction in the size.
Thus, the correct order of size is Al>Al+>Al2+>Al3+.
Hence, the answer is the option (1).
Example.4 Mendeleev’s periodic table is based on:
1) Atomic number
2) (correct)Atomic weight
3) Ionization enthalpy
4) None of the above
Solution
Mendeleev arranged the elements in horizontal rows and vertical columns in his table in order of their increasing atomic weights. In this way, elements with similar properties occupied the same vertical column.
Hence, the answer is the option (2).
Example 5: The element that does not belong to the same period of the remaining elements (modern periodic table) is:
(1) Palladium
(2) Iridium
(3) Osmium
(4) Platinum
Solution:
Palladium $\Rightarrow 5^{\text {th }}$ period
Iridium, Osmium, Platinum $\Rightarrow 6^{\text {th }}$ Period
Hence, the correct answer is option (1).
Practice More Questions from the link given in the Table:
| Modern periodic table Practice question and MCQ |
| Mendeleev’s Periodic table Practice question and MCQ |
Also, check-
Frequently Asked Questions (FAQs)
Bismuth-209, with 83 protons, was long considered the heaviest stable isotope. However, it was discovered to be slightly radioactive with an extremely long half-life. This discovery challenged our understanding of nuclear stability at high atomic numbers.
Tin, with atomic number 50, sits at the border between metals and metalloids on the periodic table. It exhibits properties of both, illustrating how atomic number influences an element's position and properties in the periodic table.
Each element has a unique atomic emission spectrum due to its specific electron configuration, which is determined by its atomic number. When excited electrons return to lower energy levels, they emit light at characteristic wavelengths, creating a "fingerprint" for each element.
Elements with even atomic numbers are often more abundant due to the greater stability of nuclei with even numbers of protons and neutrons. This phenomenon, known as the Oddo-Harkins rule, is related to nuclear binding energies and the processes of nucleosynthesis.
Electron shielding increases with atomic number as more inner electrons shield outer electrons from the nuclear charge. This concept is crucial in understanding trends in atomic size, ionization energy, and other periodic properties.
Element 118 (Oganesson) is currently the highest atomic numbered element synthesized and the last element that fits in the traditional periodic table structure. Beyond this, new elements would require a new period and potentially new groups, challenging our current understanding of element organization.
Diagonal relationships exist between certain elements with similar properties but different atomic numbers, like lithium and magnesium. These relationships arise from a balance between atomic size and electronegativity, both influenced by atomic number.
Transition metals have similar properties due to their partially filled d-orbitals, which allow for multiple oxidation states and similar reactivity. While their atomic numbers differ, their outer electron configurations are often similar, leading to comparable chemical behaviors.
Atomic numbers determine the ground state electron configuration. In transition metals, electrons can be promoted from s to d orbitals during bonding. This promotion, possible due to the energy levels determined by atomic number, explains some of their unique properties.
Metallic character generally decreases across a period and increases down a group. This trend is related to the ease of losing electrons, which is influenced by the electron configuration and effective nuclear charge, both determined by the atomic number.