Gabriel Phthalimide Synthesis, Mechanism - Reaction with FAQs
How can we efficiently synthesize primary amines from alkyl halides without unwanted side reactions? What makes the Gabriel Phthalimide Synthesis a preferred method in organic chemistry for preparing primary amines? You will understand this after reading this article on Gabriel Phtalimide synthesis. The Gabriel phthalimide synthesis is named after the German chemist Siegmund Gabriel. It is a reaction that involves the conversion of primary alkyl halides into primary amines using alkyl halides. Conventionally, the Gabriel synthesis uses potassium phthalimide.
This Story also Contains
- Gabriel Phthalimide Synthesis
- Gabriel Phthalimide reaction
- Gabriel Synthesis Mechanism
- Some Solved Examples

Gabriel Phthalimide Synthesis
The Gabriel Phthalimide Synthesis is a method for synthesizing primary amines from alkyl halides using phthalimide as a reagent. This reaction is widely used due to its ability to selectively produce primary amines, avoiding the formation of secondary or tertiary amines. The Gabriel phthalimide synthesis has applications in the alkylation of sulfonamides and imides, followed by their deprotection, to obtain amines. The alkylation of ammonia is frequently an extensive and inefficient route to amines. In the Gabriel phthalimide synthesis, phthalimide anion is recruited as a proxy of H2N−.
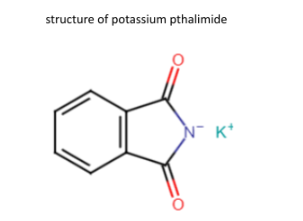
Also read -
Gabriel Phthalimide reaction
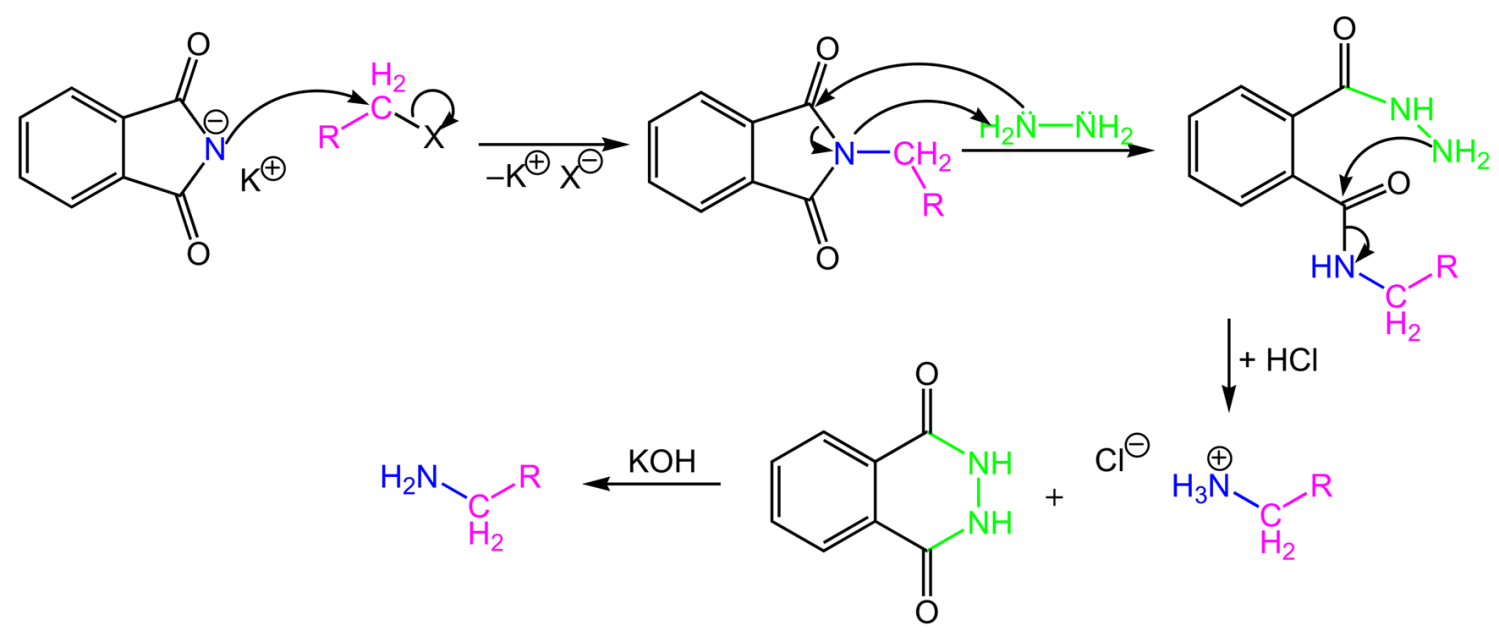
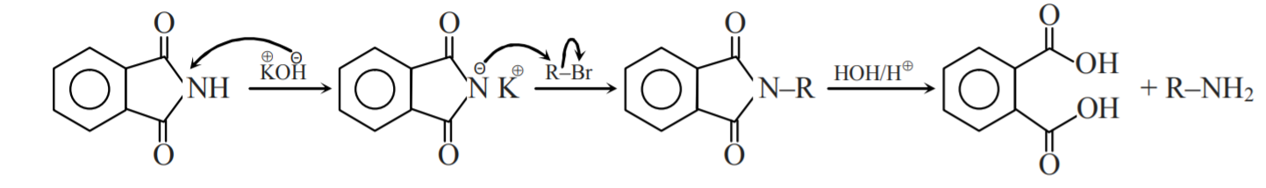
The main objective of Gabriel phthalimide synthesis is to form a primary amine (RNH2). Gabriel synthesis involves the reaction of potassium hydroxide with the phthalimide, which forms a good nucleophile in the form of an imide ion. The imide ion attacks alkyl halide via nucleophilic substitution reaction and leads to the formation of an intermediate named N-alkyl phthalimide. Phthalimide then undergoes hydrolysis, which yields a primary alkyl amine. However, aryl amines cannot be prepared through Gabriel synthesis because aryl halides do not undergo simple nucleophilic substitution. Gabriel synthesis has the advantage of eluding the possibility of overalkylation.
Before discussing the mechanism, we could write the overall Gabriel phthalimide reaction as:
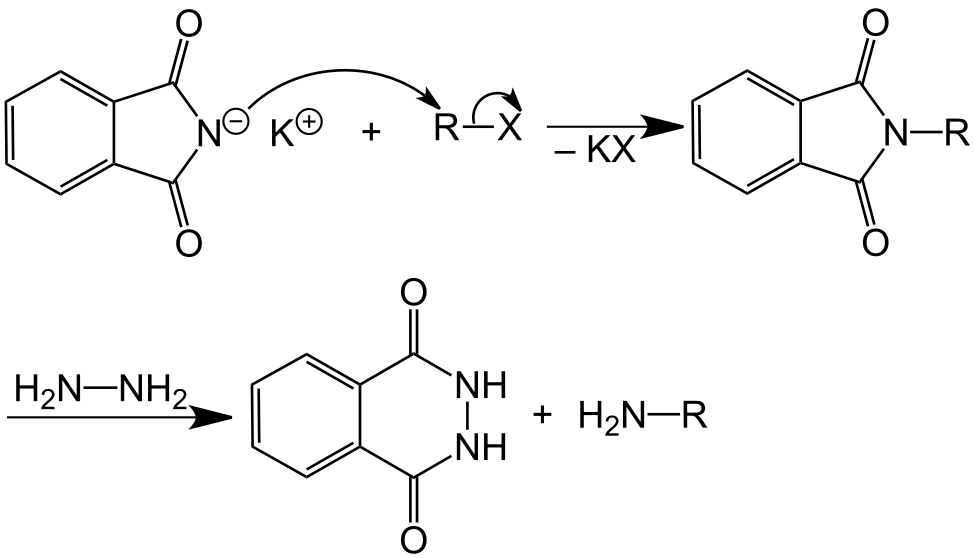
Gabriel Synthesis Mechanism
As we discussed above, the Gabriel phthalimide reaction is used for the preparation of primary amines, and the Gabriel phthalimide reaction is a nucleophilic substitution reaction. Now, we need to understand how this reaction proceeds and how different groups interact with each other to produce primary amines as the endgame. The reaction initiates with the conversion of phthalimide into potassium phthalimide by the addition of alkali base KOH or NaOH. Removal of protons from the nitrogen of phthalimide takes place; this is called deprotonation of nitrogen. In some books, this step is skipped.
.png)
Gabriel phthalimide synthesis then proceeds with potassium phthalimide. The nitrogen of potassium phthalimide gains a negative charge due to deprotonation. This negatively charged nitrogen can now act as a nucleophile and react with an alkyl halide via a bimolecular nucleophilic substitution reaction or SN2 reaction.
The nucleophilic nitrogen of Gabriel phthalimide attacks the electrophilic carbon of the alkyl halide. The potassium ion from KOH combines with the halogen of the alkyl halide and forms KX. The alkyl group of alkyl halide attaches to nucleophilic nitrogen, which results in the formation of N-alkyl phthalimide (structure given below).
.png)
Related topics link,
- Weak Acid Examples
- List of Strong Acids
- Molischs Test
- Schiff Reagent and Test
- Ronsenmund Reduction Mechanism
Structure of N-alkyl Phthalimide
At this stage of the Gabriel phthalimide reaction, there are a couple of variations for generating amines. Generally, hydrazine (NH2NH2) is used via the Ing–Manske procedure, which produces a precipitate of phthalhydrazide along with the primary amine. Processes like acidic hydrolysis or basic hydrolysis can sometimes be done. Gabriel synthesis involving acidic hydrolysis liberates an amine salt from the primary amine. We will discuss how the reaction proceeds with the addition of hydrazine. Hydrazine is a nucleophile that, on adding to carbonyl carbon, gives nucleophilic acyl substitution with N-alkyl phthalimide.
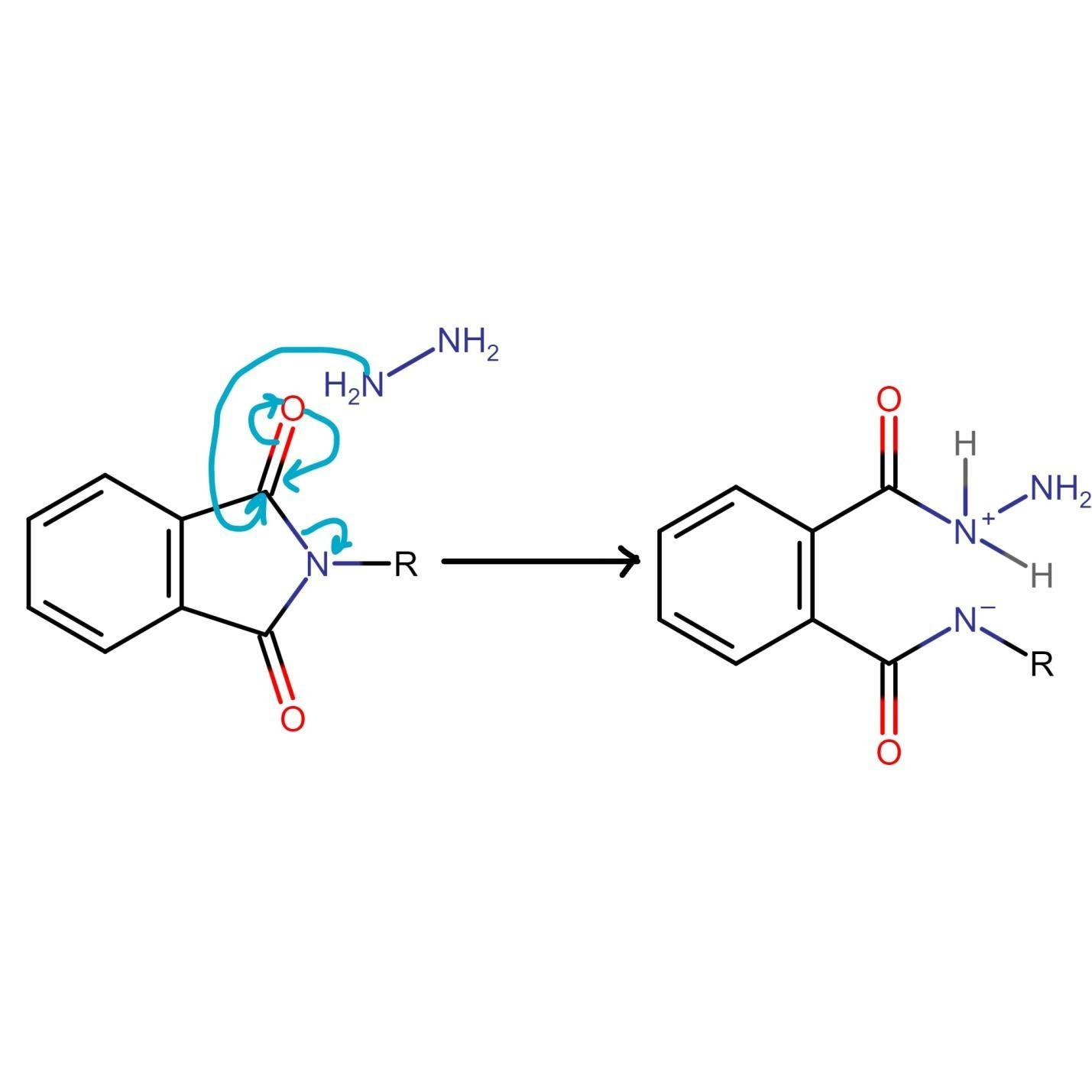
The nitrogen of phthalimide then participates in deprotonation of the protonated hydrazine.
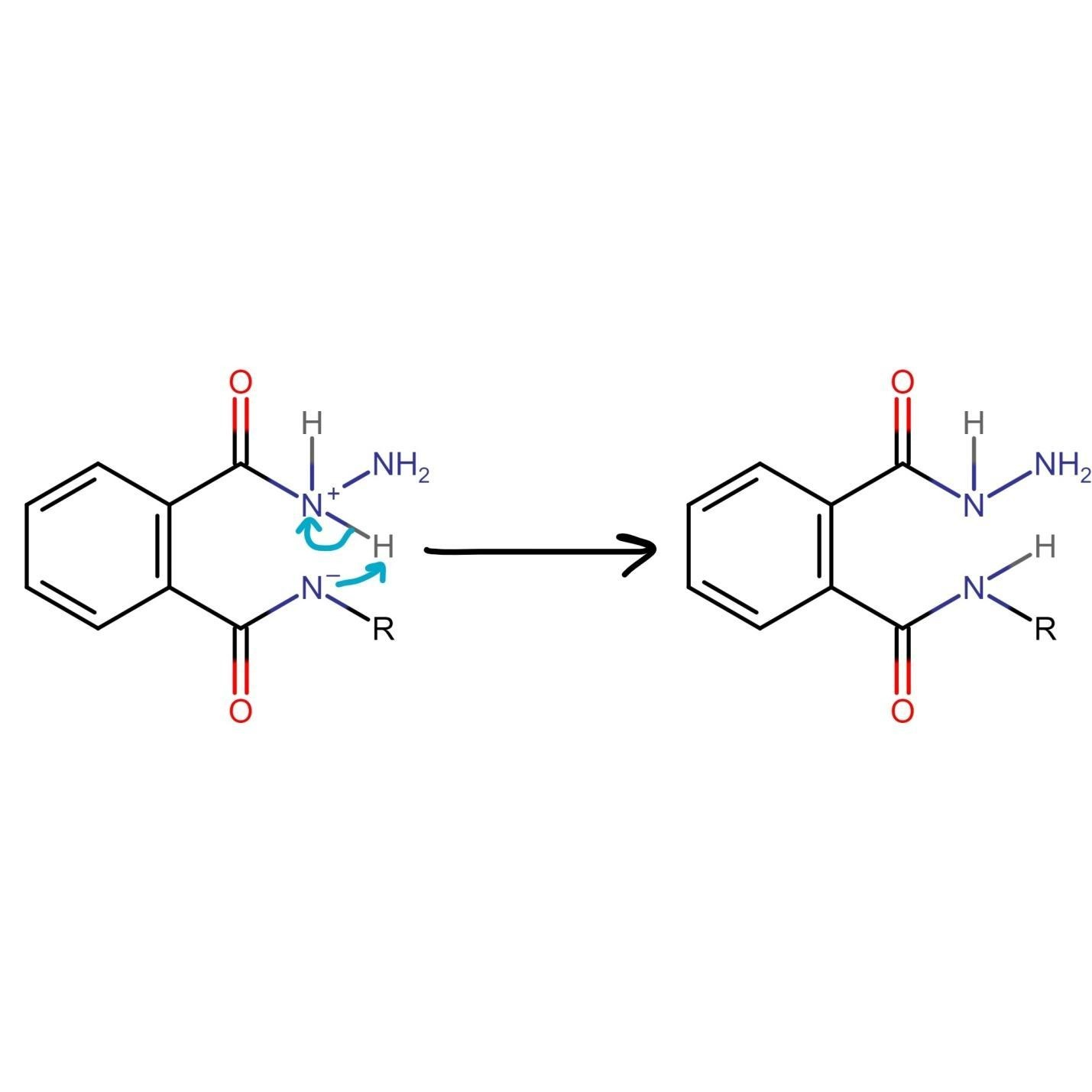
The nitrogen of hydrazine is deprotonated by the nitrogen of phthalimide, which leads to the formation of an uncharged species.
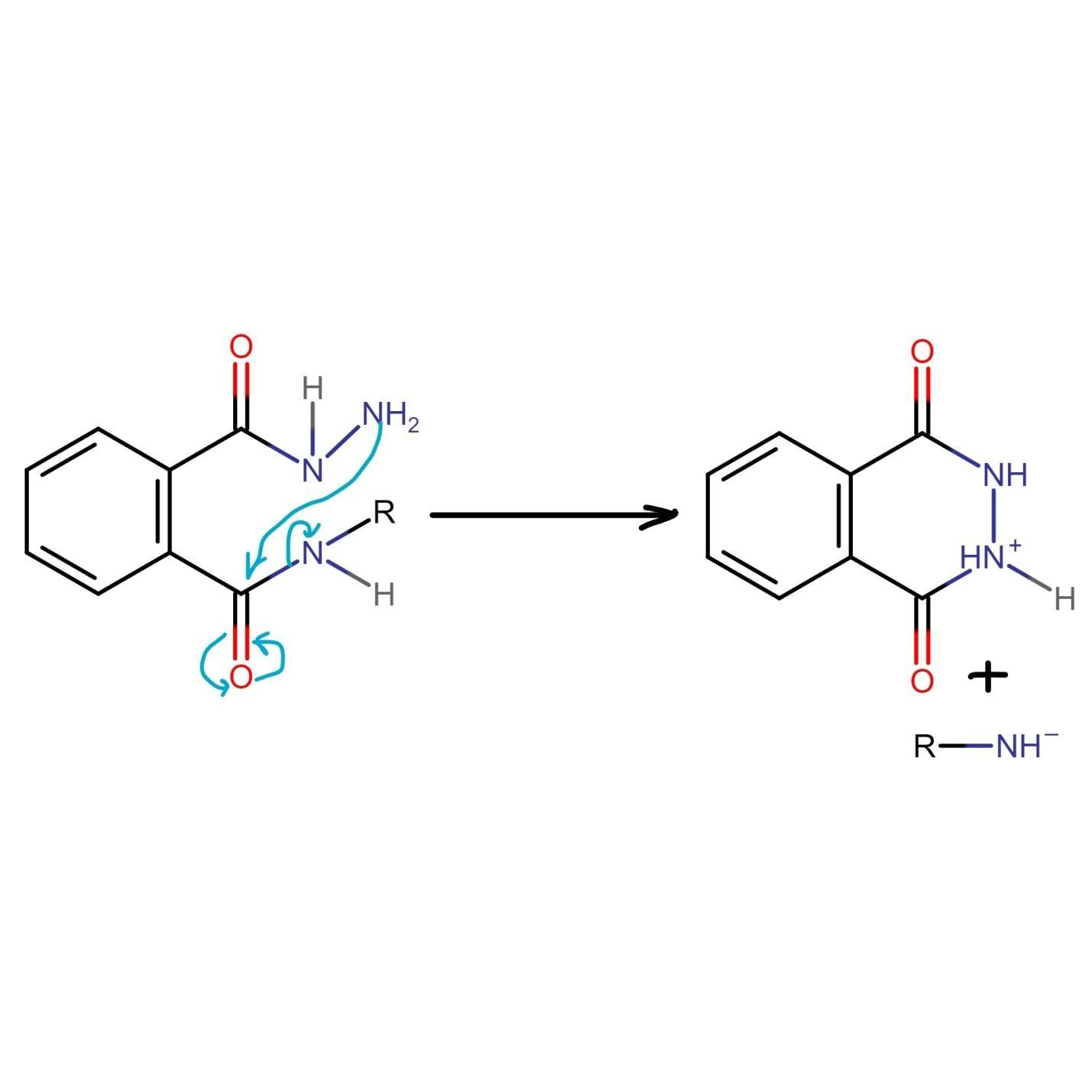
The unreacted NH2 group of hydrazine undergoes nucleophilic acyl substitution and attacks the carbonyl group liberating amine. Both species generated in this step of Gabriel phthalimide synthesis are charged which need to be taken care of.
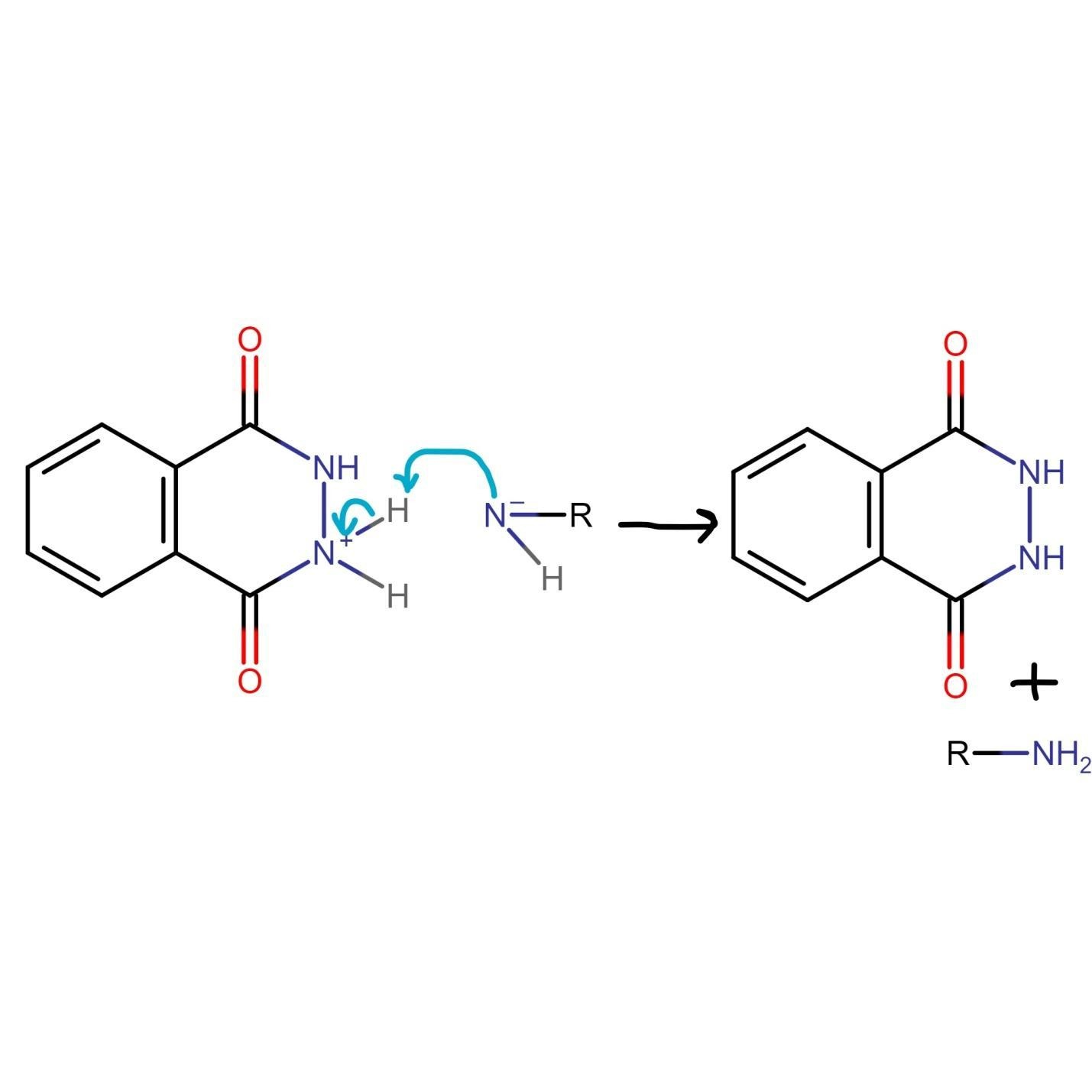
The final step of the Gabriel phthalimide reaction involves neutralization of the charge, which results in the formation of primary amine RNH2. This step marks the end of Gabriel phthalimide synthesis.
Alternate Methods of Gabriel Synthesis
Gabriel phthalimide synthesis using hydrazine often produces low yields or side products. Consequently, it is not easy to separate phthalhydrazide. This is the main problem that leads to the formulation of other methods to liberate amines from phthalimide. Alternative reagents like the sodium salt of saccharin and di-tert-butyl-iminodicarboxylate are used in place of hydrazine.
Such reagents are electronically similar to phthalimide salts and consist of ian mido nucleophile. Since Gabriel synthesis is ineffective for secondary alkyl halides, these reagents ensure the reactivity of secondary alkyl halides too. These reagents hydrolyze more readily and allow the production of secondary amines. Another common alternative includes acid hydrolysis and base hydrolysis of phthalimide.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- NCERT notes Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Gabriel Phthalimide Synthesis Involving Acid Hydrolysis
Gabriel synthesis initiates with H3O+ ion, which is made by addition of any acid in aqueous solution. The hydronium ion protonates one of the carbons of the carbonyl groups of phthalimide, and addition of water takes place, which in turn leads to the cleaving of N-alkyl phthalimide. Another molecule of nucleophilic water then attacks the other carbon of the carbonyl carbon, which develops a charge on the molecule. This extra charge forces RNH2 to detach from carbonyl carbons. The end of Gabriel synthesis is marked by the substitution of RNH2 by OH from the N-alkyl phthalimide which results in formation of an amine.
Gabriel Phthalimide Synthesis Involving Basic Hydrolysis
Gabriel phthalimide reaction initiates with an attack of OH- on one of the carbonyl carbons. Nucleophilic OH ion attacks the carbon of the carbonyl group by nucleophilic substitution reaction. This results in the cleaving of N-alkyl phthalimide. During this process, the oxygen atom of the carbonyl group gains a negative charge. Another molecule of OH ion attacks the second carbonyl group. This process of charge transfer between nucleophile and electrophile to gain stability and neutralize molecules forces RNH2 to detach from the carbonyl carbon. The nitrogen is replaced by O- ions. This marks the end of the nucleophilic substitution reaction and hence the end of the Gabriel phthalimide synthesis.
Also check-
Some Solved Examples
Question 1: Gabriel phthalimide reaction is used in the synthesis of:
1) Primary aromatic amines
2) Secondary amines
3) (correct) Primary aliphatic amines
4) Tertiary amines
Solution:
As we learn
Gabriel phthalimide row is used in the synthesis of primary aliphatic amines.
.png)
Hence, the answer is option (3).
Question 2: Which of the following compounds can be prepared in good yield by Gabriel phthalimide synthesis?
1)

2) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{NHCH}_3$
3)
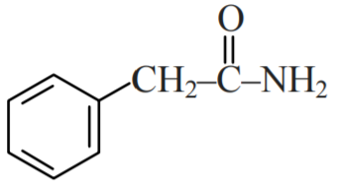
4)
Solution:
Gabriel Phthalimide Synthesis is used for the preparation of 1° Aliphatic amine from an Alkyl halide.
Thus, only Benzylamine can be prepared by Gabriel Phthalimide Synthesis

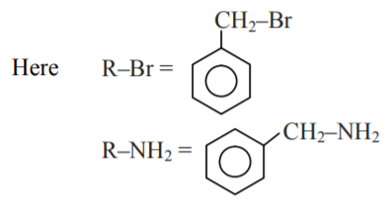
Hence, the answer is the option (1).
Question 3: Number of isomeric aromatic amines with molecular formula $\mathrm{C}_8 \mathrm{H}_{11} \mathrm{~N}_{, \text {, }}$ which can be synthesized by Gabriel Phthalimide synthesis is $\qquad$
Solution:
By Gabriel phthalimide synthesis $\rightarrow$ i-amine is prepared
$\mathrm{C}_8 \mathrm{H}_{11} \mathrm{~N} \rightarrow$ Should be aromatic & i-amine
$\begin{aligned} \text { Degree of unsaturation } & =\mathrm{C}+1-\frac{\mathrm{H}-\mathrm{N}}{2} \\ & =8+1-\frac{11-1}{2} \\ & =9-\frac{10}{2}=9-5=4\end{aligned}$
It means benzene ring
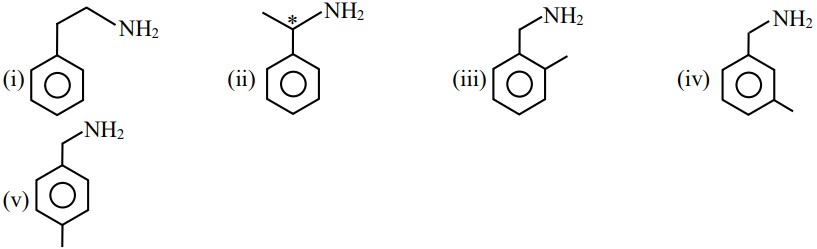
Hence, the answer is (6).
Practice More Questions With the Link Given Below
| Gabriel Phthalimide Synthesis practice questions and MCQs |
| Gabriel Phthalimide Synthesis practice questions and MCQs |
Frequently Asked Questions (FAQs)
The Gabriel synthesis is more selective for primary amines and provides cleaner yields, using phthalimide and a strong base, followed by hydrolysis. The Delépine reaction, involving trichloromethylhydrazine and alkyl halides, is less selective and can lead to side products, including secondary and tertiary amines. The Gabriel synthesis is generally preferred for its higher selectivity and efficiency.
Phase-transfer catalysts can be used in Gabriel synthesis to facilitate the reaction between the ionic phthalimide salt and the organic alkyl halide. They help transfer the phthalimide anion from the aqueous or solid phase into the organic phase where the alkyl halide is dissolved. This can increase reaction rates and yields, especially for less reactive alkyl halides.
The presence of other functional groups can significantly affect Gabriel synthesis. Electron-withdrawing groups can enhance the reactivity of the alkyl halide by making the carbon more electrophilic. However, they may also activate the molecule towards elimination reactions. Electron-donating groups generally decrease reactivity. Groups that can act as nucleophiles or electrophiles may lead to side reactions.
Yes, Gabriel synthesis can be used in the preparation of macrocyclic amines. This typically involves using a dihalide (or ditosylate/dimesylate) with a long alkyl chain. The phthalimide groups are attached at both ends, and then cyclization is achieved through intramolecular reaction. Subsequent hydrolysis yields the macrocyclic amine.
The electron-withdrawing carbonyl groups in phthalimide increase the acidity of the N-H bond. They pull electron density away from the N-H bond, making it easier to lose a proton. This increased acidity is crucial for Gabriel synthesis as it allows for easy formation of the phthalimide anion, which is the active nucleophile in the reaction.
Potassium carbonate is sometimes used in Gabriel synthesis to generate the phthalimide anion in situ. It serves as a mild base to deprotonate phthalimide without being nucleophilic itself. This can be useful when potassium phthalimide is not readily available or when milder reaction conditions are desired.
The steric bulk of the alkyl halide significantly affects the rate of Gabriel synthesis. As Gabriel synthesis proceeds via an SN2 mechanism, increased steric bulk around the reaction center slows down the reaction. Primary alkyl halides react fastest, secondary are slower, and tertiary often fail to react, instead undergoing elimination.
The reactivity order in Gabriel synthesis is typically: alkyl iodides > alkyl bromides > alkyl chlorides. This follows the general trend of leaving group ability in SN2 reactions. Iodides react fastest due to the weaker C-I bond and iodide's superior leaving group ability. Chlorides react slowest and may require longer reaction times or higher temperatures.
The carbonyl groups in phthalimide are crucial for Gabriel synthesis because:
1) They make the N-H bond more acidic, facilitating anion formation
2) They provide resonance stabilization to the phthalimide anion
3) They withdraw electron density from the nitrogen, preventing over-alkylation
4) They activate the C-N bonds for cleavage during the hydrolysis step.
Water can negatively affect Gabriel synthesis. In the alkylation step, water can hydrolyze the alkyl halide, reducing yield. It can also promote elimination reactions. In the hydrolysis step, controlled amounts of water are necessary, but excess can lead to incomplete reactions or difficulties in product isolation.

