Nucleophile - Definition, Examples, Types, FAQs
In this article we will be discussing about nucleophile, the meaning of nucleophile, definition of nucleophile, examples of nucleophile, what is meant by ambident nucleophile, examples of amident nucleophile. Here we will also discuss about some frequently asked questions related to nucleophile.
Note: In telugu the term ‘ambident’ means ‘Parisara’.
What is Nucleophilic reagents?
The term reagent means the atomic, molecular, cationic, anionic or radical species that acts upon a substrate in an organic reaction. In mechanistic treatment of organic reactions, three important types of attacking reagents are recognized. They are electrophiles, nucleophiles and free radicals.
A reagent that is electron rich by having at least one lone pair of electrons that it is willing to donate to an electron deficient substrate in a reaction is called a nucleophile or nucleophilic reagent.
Nucleophile definition or define nucleophile or define nucleophile with example: The term nucleophile literally means nucleus loving as phile is the Greek suffix for loving. These are Lewis bases which may be negatively charged ions or neutral molecules which have at least one lone pair of electrons for donation.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Some of the important nucleophile examples are:
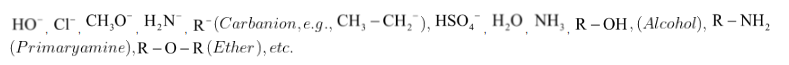
Reactions in which the attacking reagent is a nucleophile are called nucleophilic reactions.
Some of the terms that are used to explain nucleophiles are:
- Nucleophilic nature: This is used to explain the nucleophilic character of a species which shows the affinity of a species towards the positively charged nucleus.
- Nucleophilicity: This term denotes the nucleophilic strength of a species. It is generally used to compare the nucleophilic character of various nucleophiles. It is used to indicate the strength of a nucleophile.
- Nucleophilic substitution: This type of reaction occurs when a nucleophile attacks positively charged atom present in the molecule and thereby replaces a leaving group by connecting with the positively charged species.
Types of Nucleophiles
Some of the commonly used nucleophilic species are:
- Halogens – The anionic form of halogens generally acts as nucleophiles and its diatomic form does not exhibit the properties of nucleophile. For example I- acts as a powerful nucleophile in polar protic solvents whereas diatomic iodine (I2) does not behave as a nucleophile.
- Carbon – It is in organometallic reagents and enols carbon acts as nucleophiles. Carbon acts as nucleophiles in the compounds like Grignard reagents, n-butyllithium and organolithium reagents.
- Oxygen – The hydroxide ion is an example of a nucleophile containing oxygen atom and here the electron pair is donated by the oxygen atom. Alcohols and hydrogen peroxide are other examples. It is very important to note that intermolecular hydrogen bonding formation occurs in compounds containing oxygen and hydrogen. Hence nucleophilic attack does not takes place during intermolecular hydrogen bonding formation.
- Sulphur - H2S is an example for the nucleophile which contains sulphur. Because of the large size, availability of lone pair of electrons, ease in its polarization, sulphur has good nucleophilic qualities.
- Nitrogen – Nitrogen forms various nucleophiles such as ammonia, azides, amines, amides and nitrides.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
What factors determine the strength of a nucleophile?
The important factors which determine strength of a nucleophile are charge, electronegativity, steric hindrance and nature of the solvent.
- Charge – When the density of negative charge increases the nucleophilicity also increases. Thus anion is found to be a better nucleophile than a neutral molecule. Hence the conjugate base is always a good nucleophile.
Then,![]() ,
, ![]() ,
, ![]()
- Electronegativity – A highly electronegative atom acts as a poor nucleophile. Because it does not have a tendency to share its electrons. Thus as electronegativity increases the nucleophilicity decreases.
The order of electronegativity can be given as:
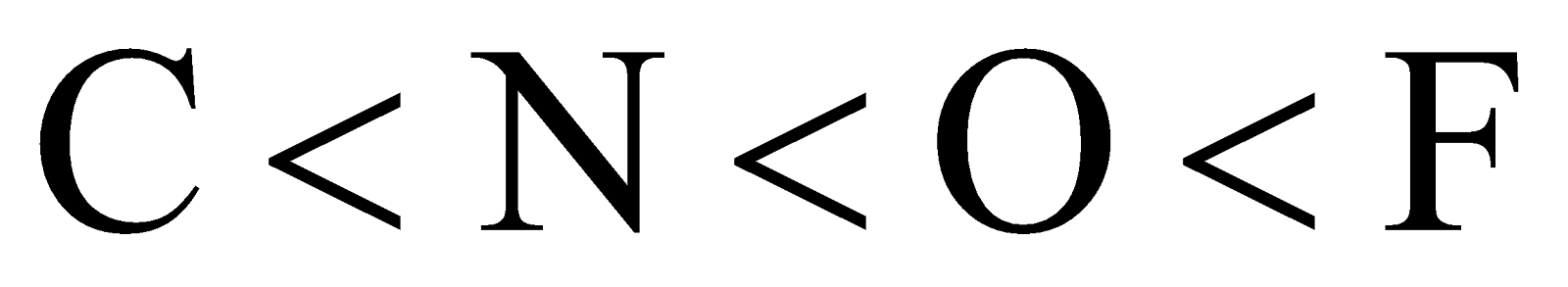
Then the order of nucleophilicity is:
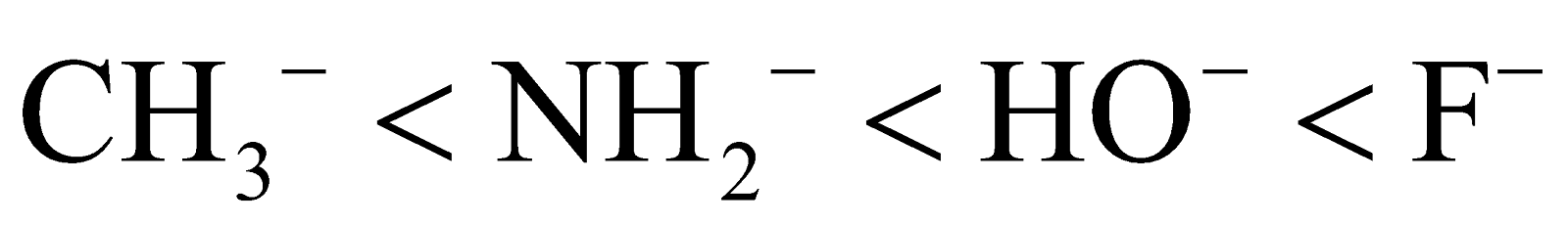
- Steric hindrance – When the nucleophile is bulky, it is very difficult to attack the substrate and thus the nucleophile becomes weaker.
Then the order of nucleophilicity is:
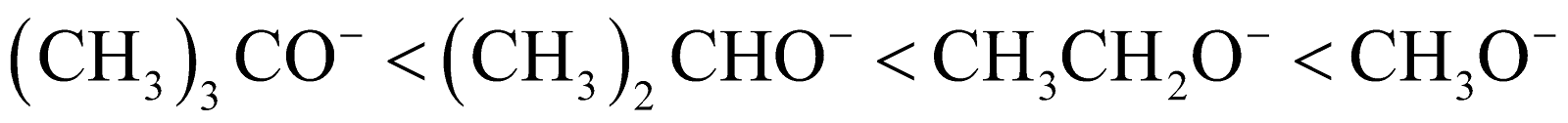
- Effect of solvent – A polar protic solvent like water or methanol can form hydrogen bond with a nucleophile. This will produce a shell of solvent molecules around the nucleophile and it inhibits the attack of the nucleophile towards the substrate and hence decreases the nucleophilicity.
A Polar aprotic solvent such as acetone or dimethylformamide solvates cations thereby leaves bare nucleophile. This increases its nucleophilicity.
What is meant by Ambident nucleophile?
An anionic nucleophile whose negative charge is delocalized over two unlike atoms can be called ambident nucleophile. The term ambident comes from two latin words ‘ambi’ which means “on both sides” and dens means “tooth”. Hence ambident nucleophile contains teeth on two sides.
Ambident nucleophiles contain two nucleophilic centres or two (-ve) sites and this negative charge is delocalized because of resonance. Hence they can attack a substrate through two sites.
Ambident nucleophile example is nitrite ion. It can attack through ‘O’ atom which results in the formation of alkyl nitrites and also it can attack through ‘N’ which gives nitroalkanes.
Cyanide and thiocyanate are also examples of ambident nucleophiles. Ambident nucleophiles can be shown as:
Nitrite ion:
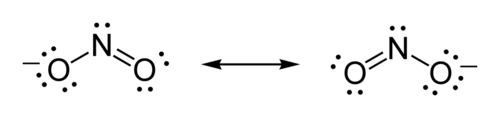
| Related topics link, |
Nitrite ion (NO2-) attack through ‘O’atom results in the formation of alkyl nitrites and it attack through ‘N’atom to form nitroalkanes.
Nitroalkane Alkyl nitrite
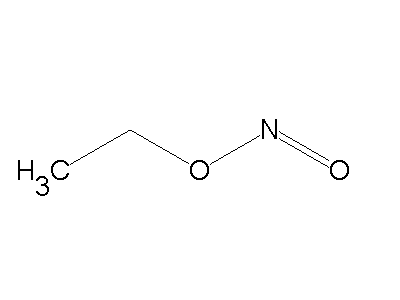
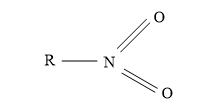
Cyanide ion:
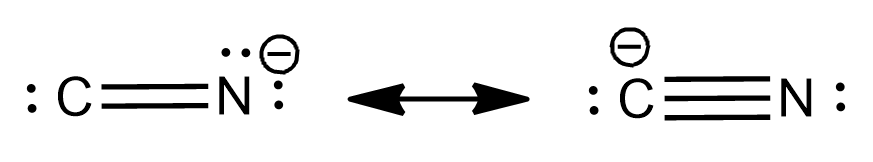
- Thiocyanate ion: Both the ‘S’ and ‘N’atoms of thiocyanate ion (SCN-) can act as nucleophiles. Alkyl halide reacts with (SCN-) through SN2 reaction to give a mixture of an alkyl thiocyanate and alkyl isothiocyanate.
- Enolate ion is an important ambident nucleophile in organic chemistry. Both the ‘C’ and the ‘O’atoms of acetone enolate can act as nucleophiles.
Nucleophilic addition
A nucleophilic addition is a reaction which involves the addition of a nucleophile to a pi-bond of a compound which results in the formation of a new sigma bond. Such type of reactions are more favoured in carbonyl compounds especially in aldehydes and ketones.
An example for nucleophilic addition reaction of acetone with HCN to give acetone cyanohydrin can be shown as:
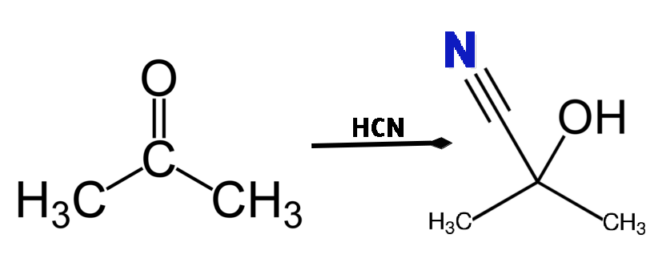
Nucleophilic substitution
A substitution reaction is one in which an electron rich nucleophile displaces the halogen atom connected to the carbon atom of an alkyl halide. The halide ion that is displaced from the carbon atom can be called leaving group.
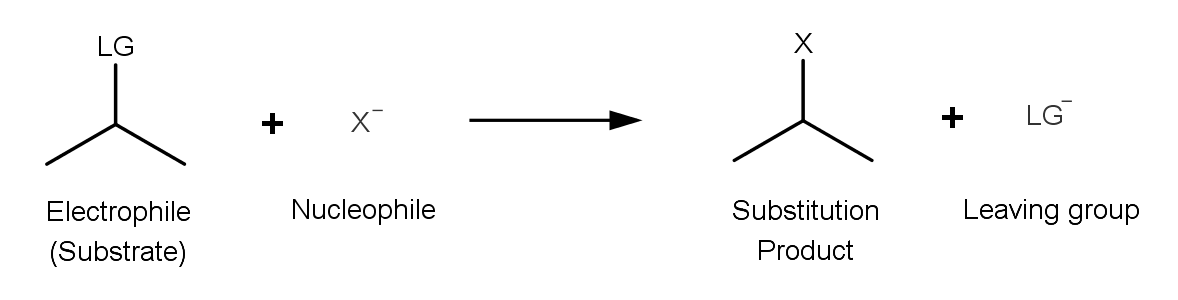
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: