Organic Chemistry - Some Basic Principles and Techniques - Notes, Topics, Formula, Books, FAQs
How do chemists know the structure of a molecule just from its reactions? How do we classify and name the thousands of organic compounds systematically? You find these answers in this chapter Some Basic Principles of Organic Chemistry. In this chapter, you'll learn fundamental ideas like how to name molecules correctly using IUPAC rules, different types of isomers, and important concepts such as inductive and resonance effects. You'll also get introduced to reagents like nucleophiles and electrophiles, which are essential when discussing organic reactions.
This Story also Contains
- Important Topics of Some Basic Principles of Organic Chemistry
- Overview of the Chapter - Some Basic Principles of Organic Chemistry
- Structural Representations Of Organic Compounds
- Classification of Organic Compounds
- Nomenclature Of Substituted Compounds
- Isomerism
- Fundamental Concepts in Organic Chemistry
- How to prepare for Some Basic Principles of Organic Chemistry
- Prescribed Books
- Previous Year Question Of Some Basic Principles of Organic Chemistry
- Conclusion

In the section on isomerism, you'll explore the different types and understand why compounds with the same chemical formula can have different physical properties. Finally, the chapter covers benzene and its derivatives, including how its substituents affect its behavior.
Important Topics of Some Basic Principles of Organic Chemistry
Organic Compounds - Classification of Organic Compounds
Organic compounds are primarily classified into open-chain compounds (aliphatic), closed-chain compounds (cyclic), and aromatic compounds. Organic compounds are further classified into into saturated, unsaturated, and functionalized compounds based on their bonding and structural features.
.jpg)
Functional Group
A functional group is a specific group of atoms in a molecule that determines its chemical properties. Examples include hydroxyl (-OH), carboxyl (-COOH), and amino (-NH₂) groups. Identifying functional groups is critical for understanding reactions and nomenclature.
Homologous Series
A homologous series is a group of organic compounds with the same functional group and a general formula, having a difference of CH₂ unit. Members of homologous series show a gradual change in physical properties but similar chemical behaviour.
IUPAC Nomenclature of Organic Chemistry
The IUPAC system provides a standardized way to name organic compounds based on their structure. IUPAC nomenclature of Organic chemistry provides a clarity in scientific communication. It involves identifying the parent chain, functional groups, and substituents.
Isomerism
Isomerism is a phenomena when compounds have the same molecular formula but different structures or spatial arrangements. It includes structural isomerism (e.g., chain, functional) and stereoisomerism (e.g., geometrical, optical).
Organic Chemistry
Organic chemistry is the study of carbon-containing compounds and their reactions. It includes natural and synthetic compounds, ranging from hydrocarbons to biomolecules like proteins and nucleic acids.
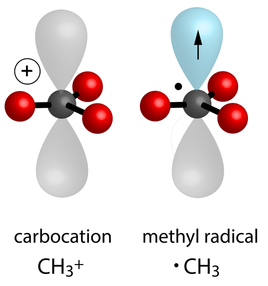
Carbocations
Carbocations are positively charged carbon species with a deficiency of electrons. Stability of carbocations is influenced by inductive, hyperconjugation, and resonance effects, and they play a key role in many organic reactions.
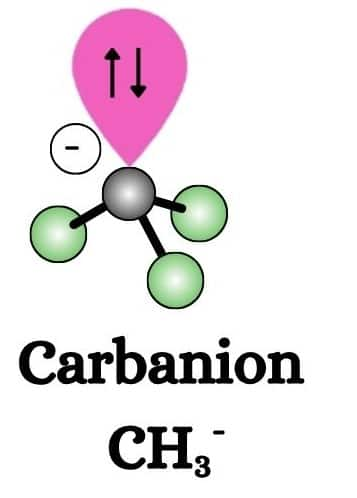
Carbanions
Carbanions are negatively charged carbon species with an excess of electrons. Carbanions are highly reactive and mostly act as nucleophiles in organic reactions.
Alkyl Free Radicals
Alkyl free radicals are neutral species with an unpaired electron on a carbon atom. Their stability of alkyl free radicals depends on hyperconjugation and resonance effects, and they are intermediates in radical reactions.
Nucleophiles and Electrophiles
Nucleophiles are electron rich species that donate electrons and electrophiles are electron deficient species that accept electrons. Nucleophiles and electrophiles interactions drive most organic reactions.
Inductive Effect
The inductive effect refers to the polarization of sigma bonds due to electronegativity differences. It influences the stability of intermediates like carbocations and acids' strengths.
Electromeric Effect
The electromeric effect is the temporary shifting of π-electrons within a molecule in the presence of an attacking reagent, crucial in explaining reaction mechanisms.
Mesomeric or Resonance Effect
The resonance effect involves the delocalization of electrons in conjugated systems, stabilizing the molecule. Mesomeric or Resonance effect explains the enhanced stability of aromatic compounds and intermediates.
Hyperconjugation
Hyperconjugation is the delocalization of sigma electrons adjacent to a positively charged carbon which provides stability to carbocations and contributing to alkene stability.
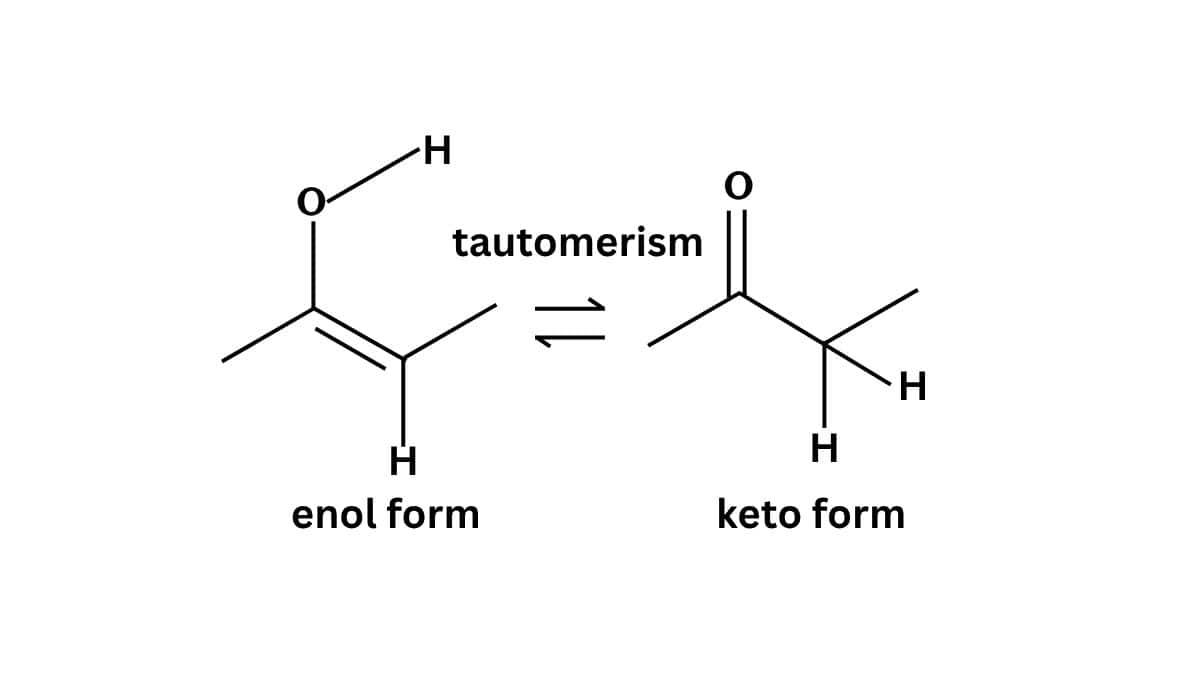
Tautomerism
Tautomerism is a reversible chemical phenomenon where compounds interconvert between isomeric forms which involves keto-enol transformations. It affects reactivity and stability.
Purification of Organic Compounds
Methods for the Purification of Organic compounds include distillation, crystallization, and chromatography, which separate organic compounds based on their physical and chemical properties.
Qualitative Analysis of Organic Compounds
Qualitative analysis identifies elements (C, H, O, N, S, halogens) in organic compounds. Techniques like Lassaigne’s test and sodium fusion test are commonly used for the Qualitative analysis of organic compounds.
Lassaigne’s Test - Test for Nitrogen, Sulphur, Halogens
Lassaigne’s test involves fusing an organic compound with sodium to detect elements like nitrogen, sulfur, and halogens through characteristic reactions.
Duma’s Method
Duma’s method is used to determine the nitrogen content in organic compounds by converting nitrogen into molecular nitrogen or ammonia for quantitative analysis. Duma’s method is an important topic for exams like NEET.
Kjeldahl’s Method
Kjeldahl’s method estimates nitrogen in organic compounds by converting nitrogen into ammonium sulfate which is followed by titration with a standard acid.
Carius Method
The Carius method quantifies halogens, sulfur, and phosphorus by oxidizing the organic compound and analyzing the resultant inorganic precipitates.
Salt Analysis
Salt analysis systematically identifies cations and anions in an inorganic salt through confirmatory tests and group-wise analysis.
Systematic Analysis of Anions
Anion analysis involves tests for acidic radicals like chloride, sulfate, nitrate, and carbonate using specific reagents and precipitation reactions. Systematic analysis of anions is an important aspect of quantitative analysis.
Systematic Analysis of Cations
Cation analysis of cations identifies basic radicals (e.g., $\mathrm{Na}^{+}, \mathrm{Fe}^{3+}$) using flame tests, precipitation, and selective reactions based on solubility and reactivity.
Overview of the Chapter - Some Basic Principles of Organic Chemistry
In this chapter, you will study the basic concepts related to organic chemistry. First, you will learn the reason due to which carbon is able to form a large number of compounds. Besides, you will learn the rules to name these organic compounds, isomerism, resonance effect, inductive effect, etc. Besides, you will also learn important tips and the best book to prepare this in the most efficient way.
Tetravalence of Carbon
Tetravalency is one of the major reason due to which carbon is able to form many compounds. While the formation of a compound, carbon forms the hybrid orbitals and because of these hybrid orbitals, these molecules have a definite shape. Other than sigma bond, carbon forms π(pi) bond as well. In this type of bonding, the hybrid orbitals are not involved but they are formed by p-orbitals. In π bond, the parallel orientation of p-orbitals occurs on adjacent carbon atoms.
Structural Representations Of Organic Compounds
Complete, condensed and Bond-line structural formulas:
In 2-dimensional geometry, carbon compound structures are represented in three ways, i.e, complete structural formula, condensed structural formula and bond-line structural formula.
(i) Complete structural formula: In this structural representation, different atoms are shown as bonded with each other via single, double or triple bond as shown in the figure.
.png)
(ii) Condensed structural formula: In this type of representation, the bonds are not shown between the atoms but the identical groups attached to an atom are represented by a subscript as shown in the figure.
.png)
(iii) Bond-line structural formula: In this type of structural representation, carbon and hydrogen atoms are not shown only the dashes or bonds are shown. Other atoms or groups like chlorine, oxygen, etc. shown.
_RvLWlAn.png)
Three-dimensional representation:
The 3-D representation of carbon compounds is also possible on paper using ‘solid and dashed wedge’ formula. In this representation, the solid wedge is used to represent the bond that is out of paper and towards the observer and dash-wedge is used to represent the bond that is away from the observer. Other bonds in the compound are in the plane of the paper and are represented simply as dash lines.
_KjOT8pn.png)
Classification of Organic Compounds
On the basis of the structures, these organic compounds are classified as follows:
.png)
Nomenclature Of Substituted Compounds
For these compounds, the following rules are followed:
(i) If one substituent is present, then the name of the substituent is prefixed to the name is Iso.
(ii) If two substituents are present, then the numbering is done in such a way that these substituents get the lowest possible numbers.
(iii) If there are three or more substituents are present then naming is done by identifying the substituent positions on the ring by following the lowest locant rule, then the direction of the numbering is done in such a way that the substituents get the lowest possible numbers. Some examples include:
Isomerism
When two or more compounds with the same chemical formula but different structures and properties are known as isomers and this phenomenon is known as isomerism.
Isomerism is of two types, viz, structural isomerism and stereoisomerism
(i) Structural isomerism: In this phenomenon, compounds have the same chemical formula but they differ in their structures. It is further classified into various categories as follows:
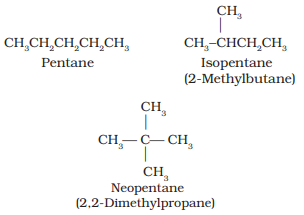
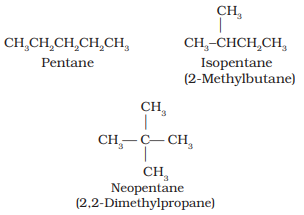
- Chain isomerism: Two or more compounds with the same chemical formula but different structures are known as chain isomers. For example, C5H12 represent three different compounds as given below.
- Position isomerism: When two or more compounds have the same chemical formula but differ in the positions of their substituent atoms or functional groups are known as position isomers. For example, C3H8 represents two different alcohols.

- Functional group isomerism: When two or more compounds have the same chemical formula but differ in the functional groups attached are known as functional group. Isomers. For example, C3H6O represents an aldehyde and a ketone.
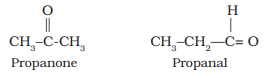
- Metamerism: In this type of isomerism, compounds differ in the type of alkyl chain attached to a functional group.
(ii) Stereoisomerism: In this class of isomerism, compounds have the same chemical formula and the same sequence of covalent bonds but differ in the relative positions of their groups. It is further classified into two groups, i,e. geometrical isomerism and optical isomerism.
Fundamental Concepts in Organic Chemistry
There are some important fundamental concepts that we need to understand carefully. These basic concepts are very useful for dealing with the organic reactions in later chapters.
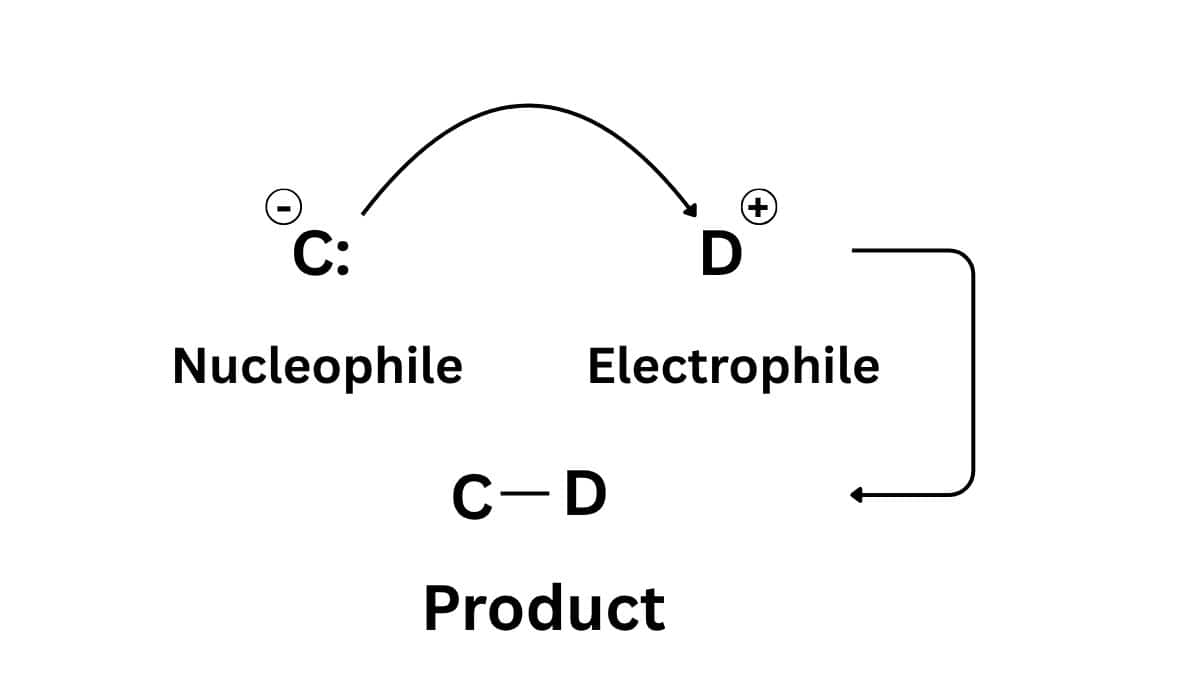
- Nucleophiles: A nucleophile is a reagent that is either negatively charged or carry lone pair of electrons. These reagents attract the protons or positive charge towards themselves.
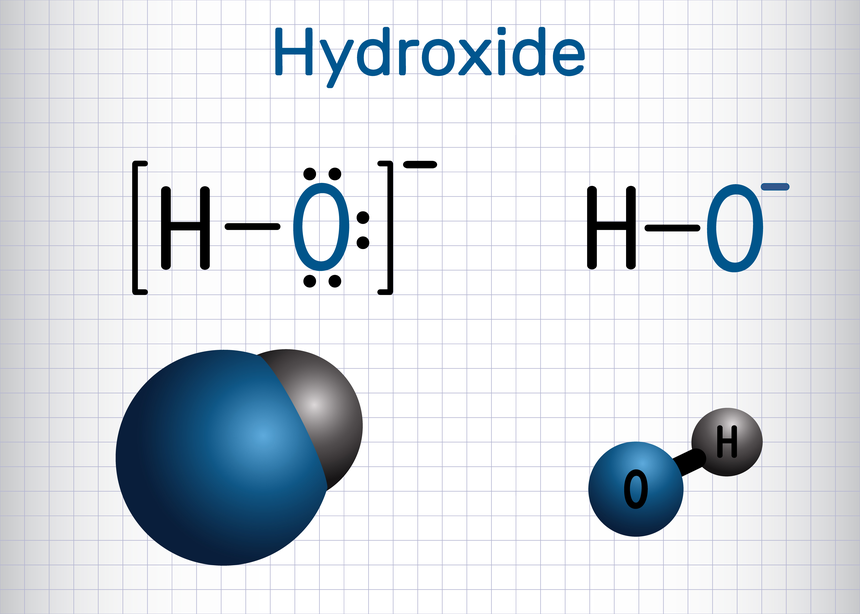
- Electrophiles: These are the reagents that are positively charged and require electrons to stabilize it.
- Inductive Effect: Inductive effect is the effect in which two different atoms of different electronegativities are bonded together. In this case, the bonded electrons are shifted towards the more electronegative atom and thus results in the polar covalent bond. When this shifting of electrons is towards the carbon then it is known as +I effect and when it is away from carbon, then it is -I effect. The relative inductive effect series based on the strength of -I effect is as follows:$-\mathrm{NH}_3^{+}>-\mathrm{NO}_2>-\mathrm{SO}_2 \mathrm{R}>-\mathrm{CN}>-\mathrm{SO}_3 \mathrm{H}>-\mathrm{CHO}>-\mathrm{CO}>-\mathrm{COOH}>-\mathrm{F}>-\mathrm{COCl}>-\mathrm{CONH}_2>-\mathrm{Cl}$$>-\mathrm{Br}>-\mathrm{I}>-\mathrm{OR}>-\mathrm{OH}>-\mathrm{NR}_2>-\mathrm{NH}_2>-\mathrm{C}_6 \mathrm{H}_5>-\mathrm{CH}=\mathrm{CH}_2>-\mathrm{H}$
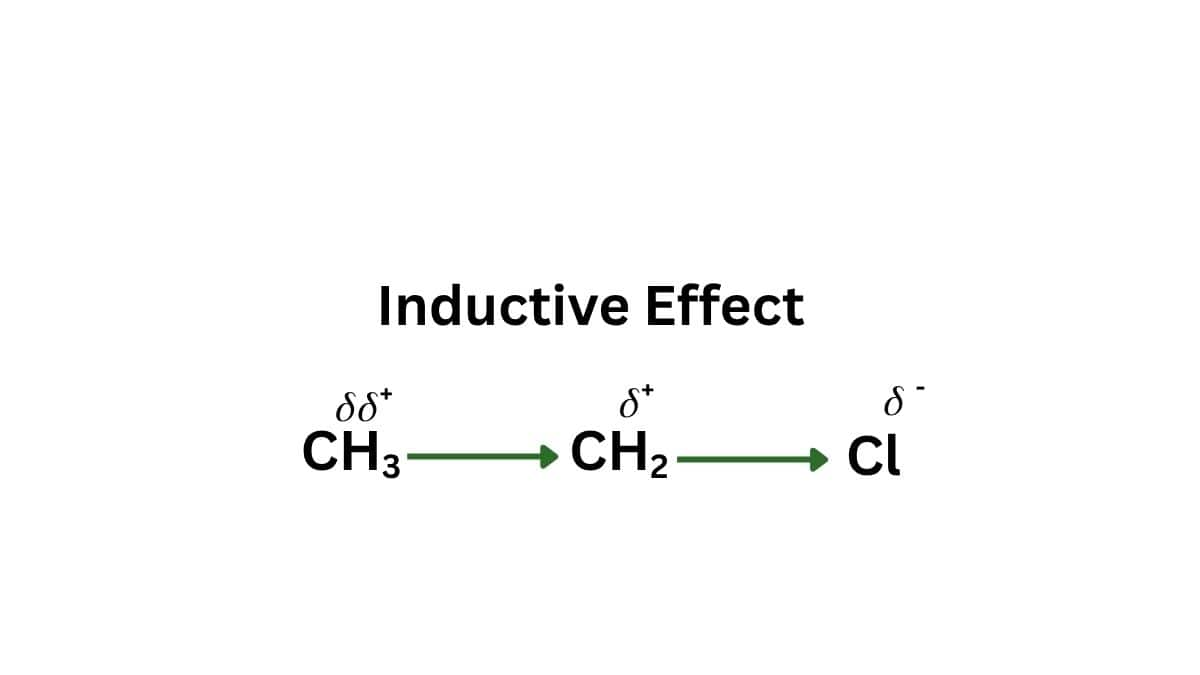
- Resonance Effect: When the polarity of a bond is created due to the interaction of two ?-bonds or between a ?-bond and a lone pair of electrons on the adjacent atom. This effect is of two types:
(i) Positive resonance effect(+R effect): In this effect, the electrons are shifted away from the substituted group attached to the conjugated system.
(ii) Negative resonance effect(-R effect): In this effect, the electrons are shifted towards the substituted group attached to the conjugated system. - Electromeric Effect: In this effect, the complete transfer of electrons takes place to one of the atoms which are directly bonded with the multiple bonds. This effect is only shown in the multiple bonds. It is temporary and becomes active only at the time of the reagent attack.
- Hyperconjugation: In this effect, delocalization of $\sigma$electrons to the unsaturated system or unshared p-orbital takes place. This effect is permanent.
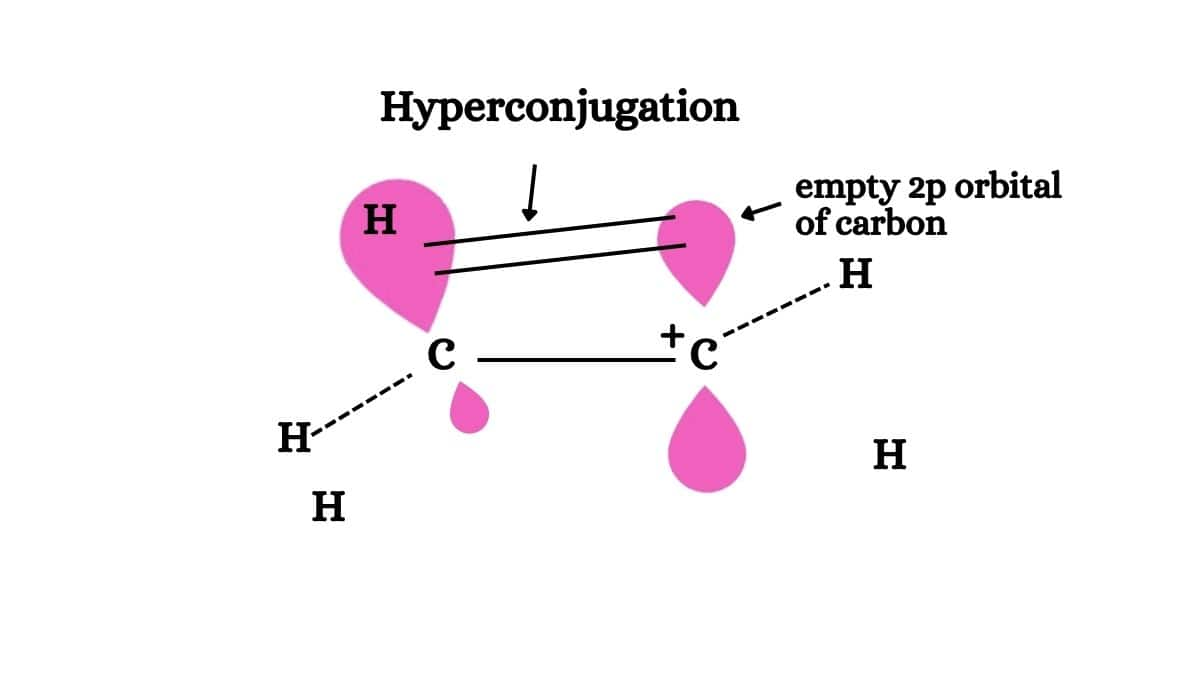
How to prepare for Some Basic Principles of Organic Chemistry
-
This is your first chapter in organic chemistry, where you'll learn all the foundational ideas and rules.
-
No prerequisites needed: You can start fresh and dive in with full focus.
-
Core ideas introduced—like resonance, isomerism, and IUPAC naming will pop up again and again in future chapters, so it's essential to understand them well.
-
Short and sweet: The chapter isn't long, but it's direct and straightforward.
-
Embrace it: Think of it as the perfect introduction—say “Yes!” with confidence.
Also read,
Prescribed Books
For this chapter, first, the NCERT book is best for initial level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - O.P Tandon. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you to provide with the variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Previous Year Question Of Some Basic Principles of Organic Chemistry
Question 1: Match the LIST-I with LIST-II
| LIST-I | LIST-II | ||
| A. | Carbocation | I. | Species that can supply a pair of electrons. |
| B. | C-Free radical | II. | Species that can receive a pair of electrons. |
| C. | Nucleophile | III. | $\mathrm{sp}^2$ hybridized carbon with empty p-orbital. |
| D. | Electrophile | IV. | $\mathrm{sp}^2 / \mathrm{sp}^3$ hybridized carbon with one unpaired electron. |
Choose the correct answer from the options given below :
1) A-IV, B-II, C-III, D-I
2) A-II, B-III, C-I, D-IV
3) A-III, B-IV, C-II, D-I
4) A-III, B-IV, C-I, D-II
Solution: Carbocation $\Rightarrow$

Nucleophile $\Rightarrow \mathrm{e}^{-}$rich species like anions etc.
$\Rightarrow$ can supply a pair of electrons
Electrophile $\Rightarrow \mathrm{e}^{-}$deficient species $\Rightarrow$ can receive
a pair of electrons.
Hence, the correct answer is option (4).
Question 2: An organic compound weighing 500 mg , produced 220 mg of $\mathrm{CO}_2$. on complete combustion. The percentage composition of carbon in the compound is________%. (nearest integer)
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1}$ of $\mathrm{C}: 12, \mathrm{O}: 16$ )
Solution:
Organic compound $\xrightarrow[\Delta]{\mathrm{CuO}} \mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
$\mathrm{n}_{\mathrm{CO}_2}=\frac{220 \times 10^{-3}}{44}=5 \times 10^{-3}$ moles
$\mathrm{m}_{\mathrm{C}}=5 \times 10^{-3} \times 12$
$\%$ m carbon $=\frac{5 \times 10^{-3} \times 12}{500 \times 10^{-3}} \times 100=12 \%$
Hence, the answer is 12.
Question 3:
Hyperconjugation involves delocalisation of ______.
(i) electrons of carbon-hydrogen $\sigma$ bond of an alkyl group directly attached to an atom of unsaturated system.
(ii) electrons of carbon-hydrogen $\sigma$ bond of alkyl group directly attached to the positively charged carbon atom.
(iii) $\pi$-electrons of carbon-carbon bond
(iv) lone pair of electrons
1) (i) and (ii)
2) (iii) and (iv)
3) (ii) and (iii)
4) None of above
Solution:
The answer is the options (i) and (ii)
Explanation: Hyperconjugation means the delocalization of electrons of the C-H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p orbital. Electrons of the C-H bond of the alkyl group enter into the partial conjugation with the attached unsaturated system or with the unshared p orbital. Thus, Hyperconjugation is a permanent effect.
Hence, the answer is option (1).
Practice more questions from the link given below:
| Inductive Effect-Practice questions and MCQs | IUPAC Nomenclature - 2-Practice questions and MCQs |
| Mesomeric or Resonance Effect-Practice questions and MCQs | Mixed Questions - 1-Practice questions and MCQs |
Conclusion
Basic Principles of Organic Chemistry—covering hybridization, IUPAC naming, inductive/resonance effects, isomerism, and reactive intermediates—build a strong foundation for Class 11 and are vital for competitive exams like JEE Main, NEET, BITSAT, and WBJEE. In JEE Main, General Organic Chemistry and related topics together contribute around one question each (~3.3%), helping secure easy marks. These concepts also support advanced topics in Class 12 (hydrocarbons, carbonyls, amines), are crucial for understanding mechanisms in physical chemistry, and reinforce bonding and reactivity in inorganic chemistry.
Frequently Asked Questions (FAQs)
Crystallization is based on the difference in the solubilities of the compound and its impurities in a suitable solvent at different temperatures. The impure compound is dissolved in a minimum amount of hot solvent, forming a saturated solution. Upon cooling, the pure compound crystallizes out as it becomes less soluble, while impurities (either more soluble or present in smaller amounts) remain in the solution.
Organic compounds are generally purified using a variety of techniques, chosen based on the properties of the compound and its impurities. Common methods include:
- Crystallization: For solids, based on differential solubility.
- Distillation: For liquids, based on differential boiling points.
- Sublimation: For solids that sublime, based on direct solid-to-gas transition.
- Chromatography: For mixtures, based on differential adsorption or partition.
- Differential Extraction: For separating components between two immiscible solvents.
A family of compounds with the same functional group and a general formula. Each successive member differs by one CH₂ unit.
Functional groups determine the chemical properties and reactivity of organic compounds. Identifying functional groups is essential for predicting reaction behaviour and naming organic molecules.
Tautomerism is the equilibrium between two isomeric forms of a compound, typically keto and enol forms. It is observed in compounds like acetylacetone and affects reactivity and stability.
The main reactive intermediates are carbocations, carbanions, and free radicals. Their stability and reactivity depend on electronic effects like inductive and resonance effects.
Lassaigne’s test is used to detect elements like nitrogen, sulphur, and halogens in organic compounds. It involves converting these elements into inorganic ions that can be identified through chemical reactions.
Purification methods include distillation, crystallization, sublimation, chromatography, and solvent extraction. The choice of method depends on the compound's physical and chemical properties.
Hybridization (sp, sp², sp³) influences molecule shape and bond strengths.
Resonance spreads out electrons across multiple atoms, stabilizing molecules.
Inductive effect is when electron density shifts through bonds due to differences in electronegativity.

