Structure of Benzene - Discovery, Lewis Structure, Properties, FAQs
Have you ever wondered why benzene, a simple hydrocarbon, is so stable despite having alternating double bonds? Why doesn’t it behave like other alkenes in chemical reactions? You can answer these questions after studying this article on the Structure of benzene. Benzene has a ring structure with six carbon atoms arranged in a planar hexagonal ring. The structure of benzene $\left(\mathrm{C}_6 \mathrm{H}_6\right)$ is unique, with each carbon atom being $s p^2$-hybridized, forming three sigma $(\sigma)$ bonds, two with adjacent carbons and one with hydrogen
This Story also Contains
- Structure of Benzene
- Discovery of Benzene
- Properties and Occurrence of Benzene
- Uses of Benzene
- Some Solved Examples
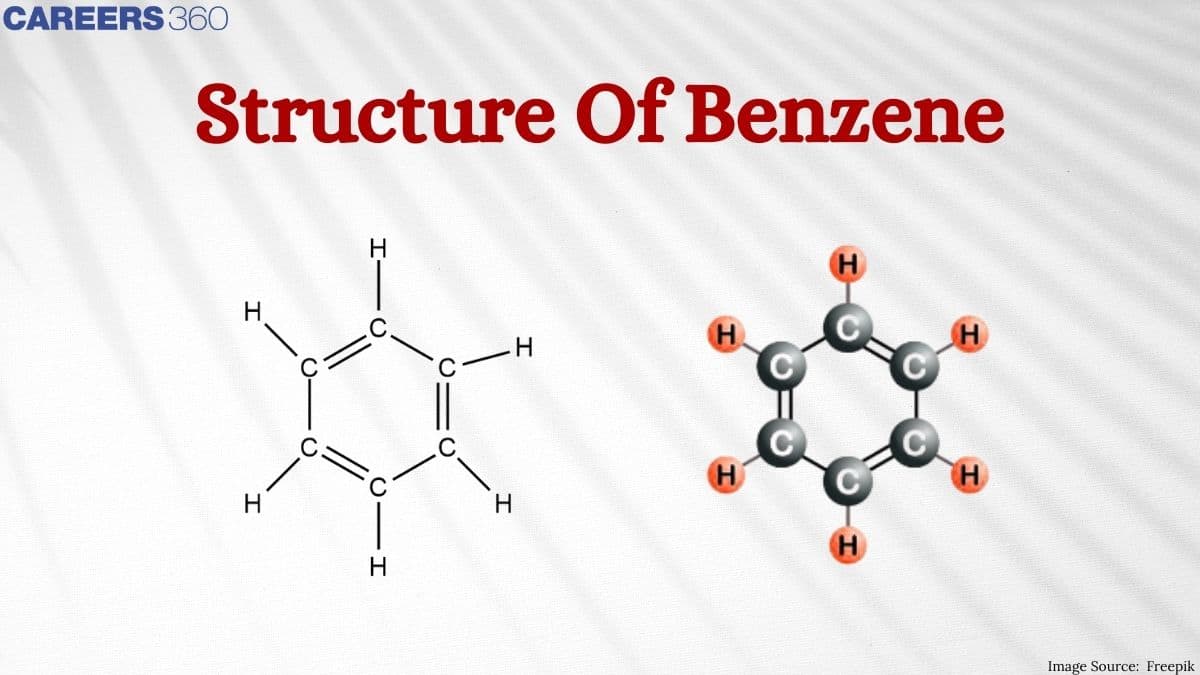
Structure of Benzene
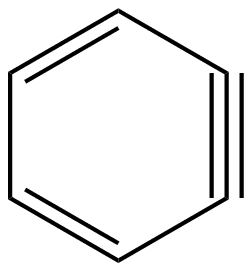
Benzene is said to be an organic chemical compound containing carbon and hydrogen. The molecular formula of benzene is $\mathrm{C}_6 \mathrm{H}_6$. This suggests that benzene is composed of six carbon atoms and six hydrogen atoms. These atoms are joined together in a planar ring, and to each carbon atom, one hydrogen atom is attached. The benzene structural formula can be drawn in the following manner:
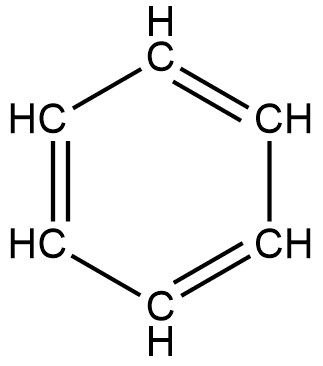
In an alternate position, it contains double bonds; this double bond shows that benzene is unsaturated in nature. Those compounds which contain double or triple bonds are said to be unsaturated in nature while those that have only a single bond are saturated in nature. Benzene is also kept in the category of hydrocarbons; these compounds are made up of carbon and hydrogen only.
The benzene formula or benzene chemical formula is $\mathrm{C}_6 \mathrm{H}_6$.
$\mathrm{C}_6 \mathrm{H}_6$, the chemical name is benzene.
Discovery of Benzene
Benzene was discovered by European pharmacists in the 16th century and the word benzene is basically derived from the word gum benzoin or we can call it benzoin resin, which is known as aromatic resin. Aromatic compounds have ring-like structures. Benzene is said to be aromatic in nature and the aromaticity concept can also be explained with the help of Huckel’s rule this rule states that a compound that contain $(4 n+2) \pi$electrons is aromatic in nature, In the case of benzene n = 1 and it contains $6 \pi$ electrons i.e. aromatic in nature.
Structure
Benzene structure contains six carbon bonds and six hydrogen bonds with alternating double bonds. According to X-ray diffraction, all six carbon-carbon bonds are of equal length, and it is measured to be 140 picometers. There is a slight difference between double and single carbon-carbon bonds. The C-C double bond is greater in length as compared to a single C-C bond. This difference can be explained on the basis of delocalization as in the case of a double bond, electrons are equally distributed to all six carbon atoms.
Benzene and cyclohexane have almost similar structures; the only difference is the loss of one hydrogen per carbon in the ring of delocalized electrons, which makes it a different kind of cyclohexane. The shape of the molecule is said to be of a planar nature. The molecular orbital of benzene generally involves the formation of three delocalized $\pi$ electrons which revolve around all six carbon atoms and gives resonating structures. Resonating structures involve the revolution of double bonds, and they give two stable resonating structures. The resonating structures of benzene can be shown as follows:
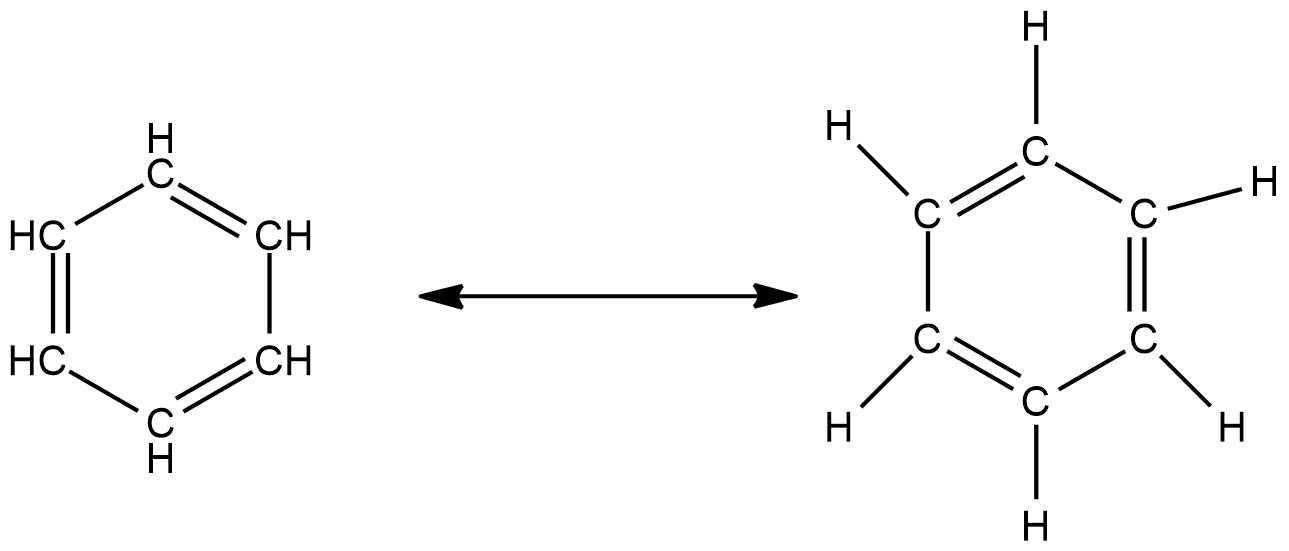
It is said to be highly stable in nature and due to its chemical properties, these are aromatic in nature. The nature of the bonding of benzene is exactly described with the cyclic hexagonal shape of six carbon atoms.
Also Read:
NCERT solutions for Chapter 12 Chemistry is provided here along with the Exemplar and Notes. These sources can be of great help for the Board as well as competitive exams like IIT-JEE and NEET.
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Lewis Structure of Benzene
The Lewis structure of any compound is shown by the valence shell electrons of that molecule. It generally tells us the arrangement of electrons in a molecule with the help of dot representation and can also be known as electron dot structure. In this type of structure, each bond is shown with the help of two dots between two atoms.
Lewis structure of benzene can be derived by using a number of steps given follow:
1. First step generally involves the determination of the total number of valence electrons of every atom present in benzene and it can be calculated by combining the valence electrons of carbon and hydrogen.
Valence electrons are those electrons that are present in the valence shell of that atom.
Number of carbon atoms in benzene = 6
Valence electrons in carbon = 4
Carbon has atomic number 6 so electron distribution in K, L shell is 2, 4 and in the outermost shell L shell which is its valence shell contains 4 electrons which correspond that it has valency 4.
Number of hydrogen atoms in benzene = 6
Valence electrons in hydrogen = 1
An atomic number of hydrogen is 1 so it contains valence electrons in the outermost shell and has valency 1.
Now, the Total number of the valence electrons in carbon = $6 \times 4=24$
Total number of the valence electrons in hydrogen = $6 \times 1=6$
2. Now determine the total number of valence electrons in benzene
Total number of valence electrons in benzene = Total number of valence electrons in carbon + Total number of valence electrons in hydrogen
= 24 + 6 = 30 electrons.
3. Step 3 involves the need for electrons to complete their octet
In the case of carbon 6 electrons are divided into two subshells K and L, subshell K contains 2 and electrons and L contains 4 electrons, here in this case K subshell is already filled and L have 4 electrons this corresponds that L subshell needs 4 more electrons to complete its octet. In the case of a hydrogen atom, it contains only 1 electron which is filled in K subshell and it contains at most 2 electrons so hydrogen needs only 1 electron to complete its octet. This corresponds to each carbon atom forming a single bond with one hydrogen atom.
Electron Dot structure of benzene can be shown as:
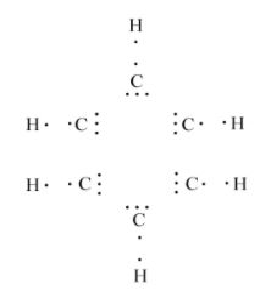
In this case, hydrogen atoms are paired but each carbon atom will need 3 more electrons for its outermost shell.
4. Number of electrons needed to acquire stable configuration
Now, according to the structure given in point 3, it is clear that each carbon atom needs 3 more electrons to complete its octet and there are 6 carbon atoms in benzene so as to total it needs 18 electrons to attain a stable configuration. This corresponds to the remaining electrons being placed in such a manner that it completes the octet of a carbon atom. The final dot structure of benzene can be shown in the following manner:
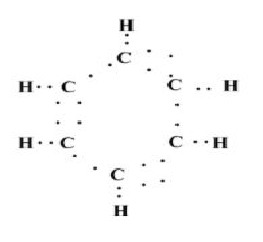
Like in this case 2 dots correspond to a single bond while 4 dots correspond to double bond atoms. This gives us the true structure of the benzene atom which contains alternative double bonds.
| Related topics link, |
5. Lewis structure of benzene
This information tells us that benzene contains six carbon atoms attached in a planar ring with alternate double and single bonds and each carbon is attached to one hydrogen atom with the help of a single bond. $\mathrm{C}_6 \mathrm{H}_6$structure of benzene can be shown as:
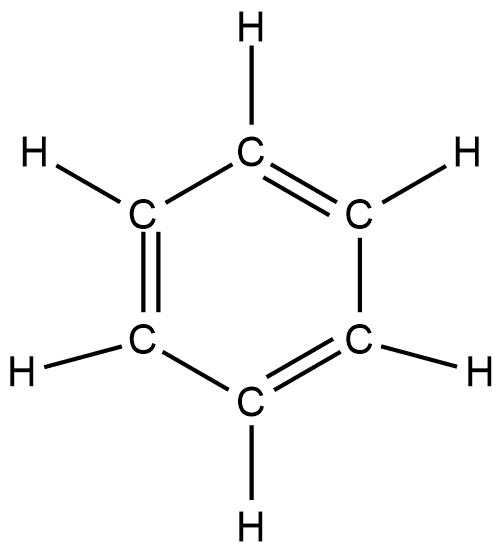
We can also show this structure in three-dimensional form which can be shown as:
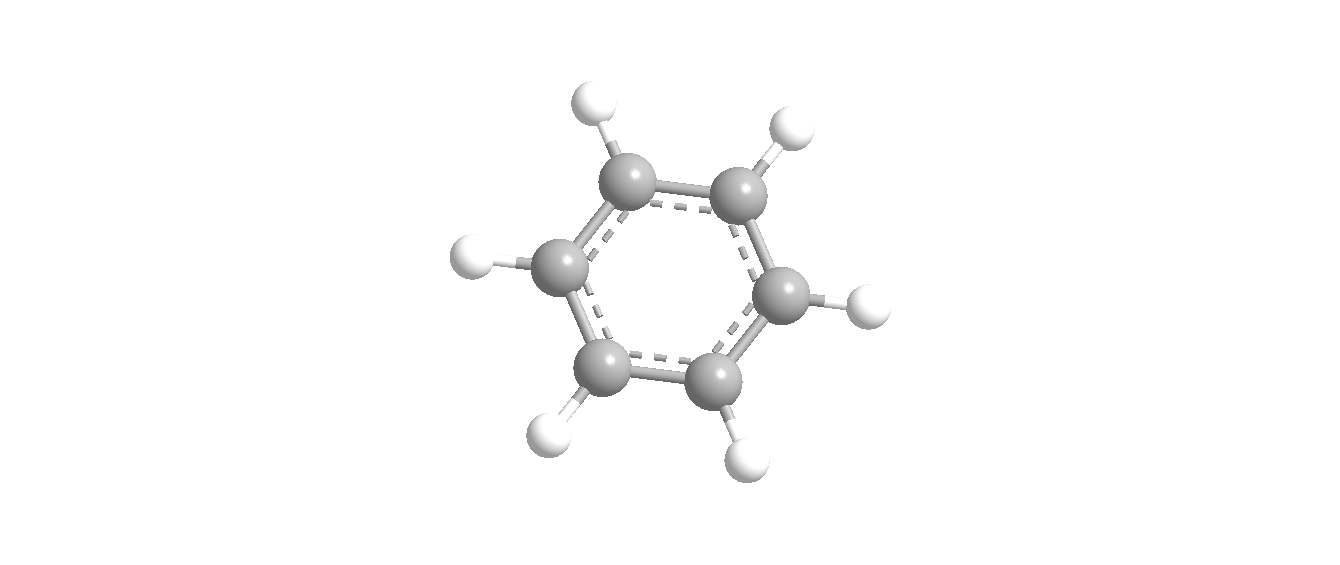
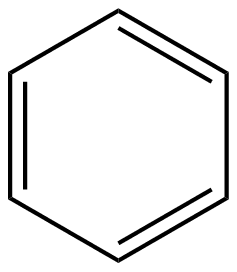
This can also be shown as a normal aromatic ring or like cyclohexane structure with the presence of double bonds this can be shown as:
Commonly Asked Questions
Benzene's discovery in 1825 by Michael Faraday was significant because it challenged existing theories about organic compounds. Its unique structure and properties led to the development of aromatic chemistry, revolutionizing our understanding of carbon-based molecules.
Benzene's structure was puzzling because its empirical formula (CH) suggested it should be highly unsaturated and reactive. However, it displayed unusual stability and didn't behave like typical unsaturated compounds, leading to confusion about its true structure.
August Kekulé reportedly had a dream of a snake biting its own tail, which inspired him to propose the cyclic structure of benzene. This dream led to the concept of a hexagonal ring of carbon atoms, a breakthrough in understanding benzene's structure.
The 4n+2 rule (Hückel's rule) predicts that cyclic, planar molecules with 4n+2 π electrons will be aromatic and thus unusually stable. It explains why benzene (6 π electrons) is aromatic, while cyclobutadiene (4 π electrons) is not.
Benzene's unique structure makes it a versatile starting material in industrial chemistry. Its aromatic character and ability to undergo various substitution reactions make it a key precursor for pharmaceuticals, plastics, dyes, and other materials.
Properties and Occurrence of Benzene
Benzene is said to be highly flammable in nature. It is said to be a volatile compound and has a gasoline-like smell. It can be found in the oil-refining process as a side product along with crude oil. It can also be found by naturally occurring substances like a forest fire which is present in plants and animals. It is a clear and colorless liquid. The molar mass of benzene is 78.11 g/mol and slightly soluble in water but easily soluble in organic solvents. Its density is less than water.
Commonly Asked Questions
Benzene is more stable due to resonance stabilization. The delocalization of π electrons across all six carbon atoms lowers the overall energy of the molecule, making it more stable than a hypothetical cyclohexatriene structure.
Aromaticity is a property of cyclic, planar molecules with delocalized π electrons. Benzene is the quintessential aromatic compound, exhibiting enhanced stability, a planar structure, and following Hückel's rule (4n+2 π electrons).
Benzene's C-C bond length (1.39 Å) is intermediate between typical single (1.54 Å) and double (1.34 Å) bonds. This uniformity in bond length is evidence of electron delocalization and equivalent bonds in the ring.
Benzene's resonance structure, represented by two alternating forms with double bonds, explains its enhanced stability. This concept of electron delocalization is crucial in understanding aromatic compounds and their unique properties.
Hückel's rule states that planar, cyclic molecules with 4n+2 π electrons (where n is a non-negative integer) are aromatic. Benzene, with 6 π electrons (n=1), satisfies this rule and is therefore aromatic.
Uses of Benzene
Benzene has a number of industrial uses like it is used in the preparation of phenol, aniline which is further used in dyes, and dodecylbenzene in detergents. In the preparation of other chemicals like ethylbenzene, cyclohexane, alkylbenzene, nitrobenzene etc. It can also use in the manufacturing of nylon fibers.
Benzyne
Benzyne is the main substituent derived from benzene; it is said to be a highly reactive species that are derived from the aromatic ring by removal of two substituents and it contains a triple bond. The structure of the benzyne atom can be shown as follows:
The molecular formula of benzyne is $\mathrm{C}_6 \mathrm{H}_4$.
Also check-
Commonly Asked Questions
Benzene is considered the parent compound of aromatic hydrocarbons because it is the simplest aromatic ring system. Many other aromatic compounds can be derived by substituting one or more hydrogen atoms on the benzene ring.
Substituents on benzene often show different reactivity compared to those on non-aromatic rings. For example, the -OH group in phenol is more acidic than in cyclohexanol due to resonance stabilization of the phenoxide ion.
Benzene's UV-Vis spectrum shows strong absorption bands due to its conjugated π electron system. The delocalized electrons can be excited by UV light, resulting in characteristic absorption peaks that reflect its electronic structure.
Benzene's resistance to oxidation is a direct result of its aromatic stability. The delocalized π electron system is energetically favorable, making it difficult for oxidizing agents to disrupt the ring structure.
In substituted benzene rings, resonance structures can show the delocalization of electrons from electron-donating groups into the ring or from the ring into electron-withdrawing groups, affecting the reactivity and properties of the compound.
Some Solved Examples
Question 1: Which one of the following correctly explains why all C–C bond lengths in benzene are equal?
A) Benzene is alicyclic and non-planar
B) Benzene contains three single and three double bonds
C) Benzene undergoes rapid oscillation between two Kekule forms
D) π-electrons in benzene are delocalized over the entire ring
Solution:
X-ray diffraction shows all six C–C bonds equal (≈1.39 Å). This is due to delocalization of $\pi$-electrons forming a resonance hybrid, not because of alternation or oscillation.
Hence, the correct answer is option (d)
Question 2: The heat of hydrogenation of benzene is 208 kJ/mol. For three isolated double bonds, it would be 360 kJ/mol. What is the approximate resonance stabilization energy of benzene?
A) 152 kJ/mol
B) 360 kJ/mol
C) 208 kJ/mol
D) 568 kJ/mol
Solution:
Resonance energy = Expected (isolated) – Observed
= 360 – 208 = 152 kJ/mol.
Hence, the correct answer is option (a)
Question 3: Which statement is incorrect regarding benzene?
A) Benzene is planar and cyclic.
B) Benzene follows Huckel’s rule ($4 n+2 \pi$ electrons).
C) Benzene undergoes electrophilic substitution more easily than addition.
D) Benzene has localized double bonds.
Solution: Benzene has delocalized π-electrons, not localized double bonds. Statements A, B, C are correct.
Hence, the correct answer is option (d)
Question 4:
The number of $\sigma$ and $\pi$ bonds in benzene is:
A) $6 \sigma$ and $6 \pi$
B) $12 \sigma$ and $3 \pi$
C) $18 \sigma$ and $3 \pi$
D) $12 \sigma$ and $6 \pi$
Solution:
-
- 6 C-C $\sigma$ bonds
- $6 \mathrm{C}-\mathrm{H} \sigma$ bonds $\rightarrow$ total $12 \sigma$
- $3 \pi$ bonds (delocalized)So, $12 \sigma$ and $3 \pi$.
Hence, the correct answer is option (b)
Frequently Asked Questions (FAQs)
Hyperconjugation in substituted benzenes involves the interaction of σ-bonding electrons (usually from alkyl groups) with the π system of the ring. This can affect the electron distribution and reactivity of the aromatic system.
Understanding benzene's structure is crucial in drug design and medicinal chemistry because many drugs contain aromatic rings. The electronic properties, reactivity, and metabolic stability of these aromatic moieties significantly influence a drug's efficacy and pharmacokinetics.
The induced ring current in benzene creates a diamagnetic anisotropy. This means that benzene's magnetic susceptibility is different parallel and perpendicular to the ring plane, a property that can be observed in various spectroscopic techniques.
The concept of aromaticity extends to PAHs, which consist of fused benzene rings. These compounds exhibit enhanced stability due to extended π electron delocalization across multiple rings, influencing their chemical and physical properties.
While less common than electrophilic substitution, benzene can undergo radical substitution reactions. This reactivity is important in certain industrial processes and provides an alternative method for functionalizing the benzene ring.
The ring current in benzene, caused by the circulation of π electrons, creates a local magnetic field. This field opposes the external field above and below the ring plane, resulting in the characteristic downfield shift of benzene protons in NMR spectroscopy.
Benzene's delocalized π electron system allows it to act as a ligand in organometallic complexes. It can coordinate to metals in a η6 (eta-6) fashion, where all six carbon atoms interact with the metal center.
The aromaticity of benzene generally makes it less reactive in pericyclic reactions compared to non-aromatic dienes. However, under certain conditions, benzene can participate in reactions like Diels-Alder cycloadditions, often requiring harsh conditions.
Benzene can form complexes with metals (e.g., chromium tricarbonyl complexes) due to its π electron system. These complexes are important in organometallic chemistry and have applications in catalysis and materials science.
Antiaromaticity is the opposite of aromaticity, occurring in cyclic compounds with 4n π electrons. While benzene is aromatic and stable, antiaromatic compounds like cyclobutadiene are highly unstable due to their electron configuration.

