Structure of the Atom - Notes, Topics, Formula, Books, FAQs
Have you ever thought about what the building blocks of everything we see around us in our daily life, from the air we breathe to the devices we use? The answer is atom. Understanding the structure of an atom helps us explain why substances behave the way they do, why chemical reactions occur, and how matter is organized at the microscopic level. In this chapter we understand the basic terms electrons, protons, neutrons, atomic number, mass number, isotopes, isobars, velocity, frequency, wavelength, wavenumber, orbitals, quantum numbers, etc.
This Story also Contains
- Important Topics Of Atomic Structure
- Atomic Structure Overview
- Line Spectrum of Hydrogen
- Quantum Numbers
- Atomic Structure Formulas
- How To Prepare The Atomic Structure
- Previous Year Questions of the Structure of Atom
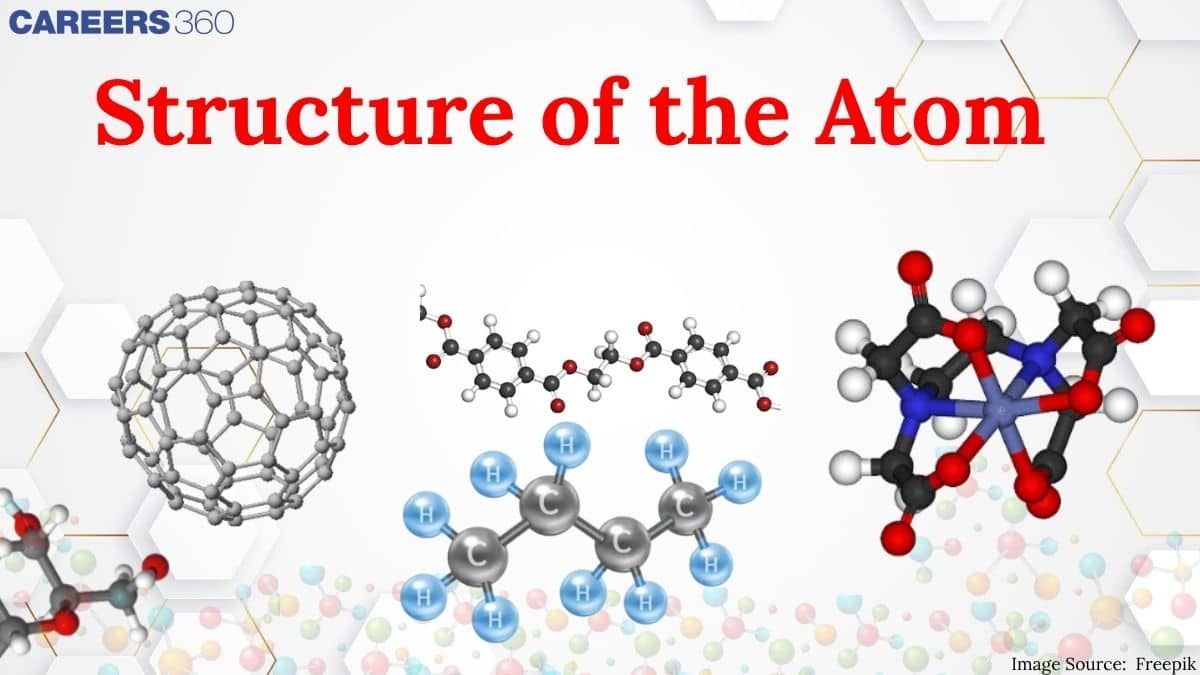
Over the years, many scientists have come across and given theories related to it. Dalton was the first to give postulates about the atoms that they were indivisible particles, but subsequent research proved that they have a complicated interior structure. Rutherford's gold foil experiment proposed the presence of a dense nucleus at the centre of the atom, whereas Thomson's model of "plum pudding" explains how electrons were contained in a positively charged sphere where positive and negative charges are disperse in a sphere. Rutherford's concept, however, was not able to explain how electrons do not collapse into the nucleus of the atom because of the opposite charges attraction The important topics of the structure of atoms class 11 are mentioned below in this article.
Important Topics Of Atomic Structure
Thomson Atomic Model
Thomson had just discovered electrons in 1897, and this was actually the first model to try speaking about the internal configuration of atoms. This is commonly referred to as the "plum pudding model.". Thomson Atomic Model has many postulates that tell about the model.
Rutherford Atomic Model And Its Limitations:
The Rutherford Atomic Model, proposed by Ernest Rutherford, had several limitations like it could not explain the stability of atoms, it did not explain how electrons are arranged inside the atom, and many more.

The observations of the Rutherford Alpha scattering experiment are
-
Most of the part of the atom is empty and atom is spherical in shape.
-
Each atom consists of a small, heavy, positively charged portion located at the centre, known as nucleus.
-
All positive charges of an atom (i.e, protons) are present in the nucleus and electrons move around the nucleus in circular orbits.
-
Electrons and the nucleus are held together by electrostatic forces of attraction.
Limitations of Rutherford’s Model
-
Does not explain stability of the atom
-
Does not explain atomic spectra
-
Rutherford’s model did not describe why electrons do not fall into the nucleus or how they maintain specific energy states.
Electromagnetic Radiation:
Electromagnetic Radiation refers to the transmission of energy through oscillating electric and magnetic fields at the speed of light
Planck's Quantum Theory:
Planck's Quantum Theory states that energy can only be absorbed or emitted by atoms and molecules in discrete quantities which is called Quanta.
Photoelectric Effect:
The Photoelectric Effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light.
Bohr's Model Of An Atom
Bohr's Model Of An Atom is a model that describes an atom as a small, positively charged nucleus surrounded by negatively charged electrons in circular orbits.
.png)
Postulates
-
Existence of Discrete Orbits (Stationary States)
-
The electron in a hydrogen atom revolves around the nucleus in certain stable circular orbits without emitting energy.
-
Each allowed orbit is called a stationary state.
-
-
Quantization of Angular Momentum
-
Only those orbits are permitted in which the electron’s angular momentum (mvr) is an integral multiple of $\frac{h}{2 \pi}$
-
$m v r=n \frac{h}{2 \pi}$
Hydrogen Spectrum:
The Hydrogen Spectrum is the line spectrum emitted by a hydrogen atom when an excited hydrogen atom returns to its ground state.
De Broglie Relationship:
The De Broglie Relationship describes the relationship between a particle's wavelength and its momentum. It was introduced in 1924 by French physicist Louis de Broglie.
Quantum Numbers:
The Quantum Numbers are those that describe the location of an electron in an atom. They determine the properties of atomic orbital and electrons in that orbital.
The four quantum numbers are:
1) Principal Quantum Number ( n )
-
Represents: Main energy level or shell (K, L, M…).
-
Values: Positive integers n=1,2,3…
2) Azimuthal / Angular Momentum Quantum Number ( l )
Represents: Subshell (shape of the orbital).
Values: $l=0,1,2 \ldots(n-1)$
- $l=0 \rightarrow \mathrm{~s}$ subshell (spherical)
- $l=1 \rightarrow \mathbf{p}$ subshell (dumbbell)
- $l=2 \rightarrow \mathrm{~d}$ subshell (cloverleaf)
- $l=3 \rightarrow \mathrm{f}$ subshell
3) Magnetic Quantum Number ( m_l )
- Represents: Orientation of the orbital in space.
- Values: From $-l$ to $+l$, including 0 .
- For $l=1$ (p subshell): $m_l=-1,0,+1$ (three orientations: $\mathrm{p}_{\mathrm{x}}, \mathrm{p}_{\mathrm{y}}, \mathrm{p} \_\mathrm{z}$ ).
4) Spin Quantum Number ( m_s )
- Represents: Direction of electron spin.
- Values: $+\frac{1}{2}$ (spin up) or $-\frac{1}{2}$ (spin down).
Radial Nodes And Planar Nodes:
Radial Nodes and Planar Nodes are the regions in an orbit where the probability of finding an electron is zero. These are the spherical surfaces around the nucleus.
Frequency, Time Period, And Angular Frequency:
Frequency, Time Period, and Angular Frequency is the important topic of atomic structure. All these are parameters of periodic motion to define it completely. Frequency is defined as the number of vibrations per second or the number of complete oscillations per second. The time period is the time taken by a particular electron to revolve in a particular orbit.
Electronic Configuration:
Electronic Configuration describes the location of an electron around the nucleus of an atom. It is written by following the standard notation.
Stability Of Orbitals: Half-filled And Completely-filled:
Stability Of Orbitals: Half-filled And Completely-filled is the topic of atomic structure in which we study the stability of orbitals as Half-filled and completely-filled orbitals are more stable than other configurations because of symmetry and exchange energy.
Atomic Structure Overview
In this chapter, the candidate will, first of all, know about the atomic theory proposed by Dalton in 1808 who regarded the atom as the indivisible particle of matter. At the end of the nineteenth century, it was proved that atoms are divisible and consist of three fundamental particles: Electrons, protons, and neutrons. Faraday then discovered electrons using a cathode ray discharge tube experiment. Neutrons were discovered by James Chadwick by bombarding a thin sheet of beryllium with alpha particles. Various atomic models were proposed to explain the structure of the atom. The aspirant will know about Thomson's plum-pudding model, Rutherford's atomic model, and Bohr's model of an atom.
Line Spectrum of Hydrogen
When an electric discharge is passed through gaseous hydrogen, the H2 molecules dissociate and the energetically excited hydrogen atoms produced emit electromagnetic radiation of discrete frequencies. These radiations are emitted because of electronic transitions upon de-excitation to different energy levels and on the basis of the final energy level of transition, the hydrogen spectrum consists of several series of lines named after their discoverers like Lyman series, Balmer Series, Paschen Series, Bracket Series, Pfund Series.
Line Spectrum of Hydrogen-like atoms
$
\frac{1}{\lambda}=R Z^2\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)
$
Where $R$ is called Rydberg constant, $R=109677 \mathrm{~cm}^{-1}, Z$ is atomic number
$n_1=$ final orbit occupied after de-excitation $=1,2,3 \ldots$
$\mathrm{n}_2=$ initial orbit occupied before de-excitation
Lyman Series spectrum:
Transition of electrons from higher orbits to $n=1$ result in the Lyman Series
$
n_1=1 \text { and } n_2=2,3,4 \ldots
$
For the H atom, this lies in the Ultraviolet region. For elements with higher $Z$, the Balmer lines lie in the Ultraviolet region
Balmer Series Spectrum:
Transition of electrons from higher orbits to $n=2$ results in the Balmer Series
Where $n_1=2$ and $n_2=3,4,5,6 \ldots$
For the H atom, this generally lies in the visible region.
Paschen, Bracket, and Pfund Series spectrums:
Transition of electrons from higher orbits to $n=3,4$ and 5 respectively, result in the Paschen, Bracket, and the Pfund Series
These lines lie in the Infrared Region for the H atom.
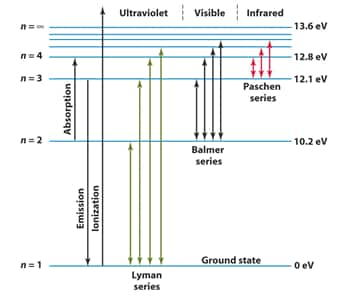
The aspirant will also learn about Planck's quantum theory, in which substances absorb or radiate energy discontinuously in the form of small packets. The phenomenon of the photoelectric effect, in which there is the ejection of an electron from the surface of a metal when light of suitable frequency strikes its surface, is something very interesting to know in this chapter. Following it, an aspirant will come across four quantum numbers like the principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number, including the shape and size of different orbitals.
Shape of s Orbital: (Spherical)
.png)
Shape of the p Orbital:(Dumbbell-shaped)
.png)
Shape of the d Orbital:
.png)
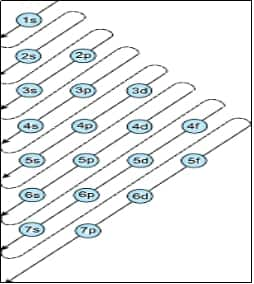
Rules for filling of electrons in various orbitals is a very important part to be studied in this chapter which will be helpful in the rest of the chemistry portion especially in inorganic and organic chemistry.
Rules For Filling Orbitals:
Rule 1 - Aufbau's principle - Lowest energy orbitals are filled first. Thus, the order of filling 1s, 2s,2p, 3s,3p, 4s, 3d, etc.
Rule 2 - Pauli Exclusion Principle - Only two electrons are permitted per orbital and they must be of opposite spin.
Rule 3- Hund's Rule - No pairing of electrons starts in any of the degenerate orbitals until all orbitals of the subshell contain one electron each with parallel spin.
Quantum Numbers
They are the set of four numbers that explain the state of an electron, i.e., location, energy, type of orbital, orientation of orbital, etc., in an atom. Various quantum numbers are as follows:
1. Principal quantum number( n)
2. Azimuthal quantum number(l)
3. Magnetic quantum number( m )
4. Spin quantum number(s)
Atomic Structure Formulas
1. The velocity of the electron in the nth Bohr orbit:
$v=2.18 \times 10^8 \frac{Z}{n} \mathrm{~cm} / \mathrm{sec}$
2. The radius of the nth Bohr orbital:
$r_n=0.529 \frac{n^2}{z} A^0$
3. The total energy of an electron in nth orbit:
$E_n=-13.6 \frac{z^2}{n^2} \mathrm{eV}$
4. The kinetic energy of an electron: -(total energy of an electron):
$13.6 \frac{Z^2}{n^2} \mathrm{eV}=-\mathrm{P} \cdot \mathrm{E} / 2$
5. The potential energy of the electron:
$-27.2 \frac{Z^2}{n^2} \mathrm{eV}$
6. Line Spectrum of Hydrogen-like atoms
$
\frac{1}{\lambda}=R Z^2\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right)
$
Where R is called the Rydberg constant, $R=1.097 * 10^7$ where $\mathrm{Z}=$ atomic number.
7. De-Broglie wavelength
$\lambda=\frac{h}{m v}=\frac{h}{p}$
Also read
| Atomic Number Mass Number | Dipole Moment |
| Anions and Cations Difference | Shapes of Orbitals |
| Matter Particles Characteristics | Bohrs Model |
| Difference Between Orbit and Orbitals |
Recommended Video on the Structure of an Atom
How To Prepare The Atomic Structure
The first and foremost thing a candidate should do is read the NCERT book thoroughly, with all the topics in this chapter. In order to get away with the confusion of "How to prepare for atomic structure," the aspirant can also take the help of animation and videos, which are easily available on the internet, to understand the shape of orbitals, Photoelectric Effect, Bohr's Model Of An Atom, and other related topics. It is very important to have a clear picture of the experimental setup and observations in the mind of the candidate. The candidate should also focus on the previous year's question papers related to atomic structure. Based on the analysis, it is found that most of the numerical problems are from Bohr's theory and the hydrogen spectrum. These are important topics froman exam point of view. So, the candidate should try to solve as many problems from these two topics.
To prepare for atomic structure, these are some reference books that should be consulted;
1. J.D. Lee
2. O.P.Tandon
Also read:
Previous Year Questions of the Structure of Atom
Example 1: When an electron jumps from $n=4$ to $n=2$, then the change in angular momentum is approximately.
1) $1.1 \times 10^{-34} \mathrm{Js}$
2)$2.2 \times 10^{-34} \mathrm{Js}$
3) $3.3 \times 10^{-34} \mathrm{Js}$
4) $4.1 \times 10^{-34} \mathrm{Js}$
Solution
The angular momentum of the electron in any $\mathrm{n}^{\text {th }}$ Bohr Orbit is given by
$
\begin{aligned}
& m v r=\frac{n h}{2 \pi} \\
& \Delta L=4\left(\frac{h}{2 \pi}\right)-2\left(\frac{h}{2 \pi}\right)=\frac{h}{\pi} \\
& \Delta L=\frac{6.626 \times 10^{-34}}{3.14}=2.2 \times 10^{-34}
\end{aligned}
$
Hence, the answer is option (2).
Example 2: Which of the following statements is incorrect for Bohr's model of an atom?
1) It is valid for a multi-electronic species
2) Angular momentum of an electron is quantized
3) The centripetal force of attraction required for circular motion is provided by the electrostatic force of attraction between the electron and the nucleus
4) Orbits have fixed energy and are referred to as stationary states
Solution:
Bohr's model is valid for only electronic species.
All other given statements given in the options are correct.
Hence, the answer is option (1).
Example 3: The electronic configurations of three elements A, B and C are given below. Answer the questions from 14 to 17 on the basis of these configurations.
A $1 s^2 2 s^2 2 p^6$
B $1 s^2 2 s^2 2 p^6 3 s^2 3 p^3$
C $1 s^2 2 s^2 2 p^6 3 s^2 3 p^5$
The molecular formula of the compound formed from B and C will be
1) BC
2) B2C
3) BC2
4) BC3
Solution:
Here, B represents phosphorus(P) and C represents Chlorine(Cl). Therefore, the compound formed will be PCl3, i.e., BC3.
Hence, the answer is the option (4).
Question 4:
Which of the following statements concerning the quantum numbers are correct?
(i) Angular quantum number determines the three dimensional shape of the orbital.
(ii) The principal quantum number determines the orientation and energy of the orbital.
(iii) Magnetic quantum number determines the size of the orbital.
(iv) Spin quantum number of an electron determines the orientation of the spin of electron relative to the chosen axis.
1) (i) and (iv)
2) (iii) and (iv)
3) (ii) and (iii)
4) None of above
Solution:
The answer is the options (i) and (iv) as only these two statements are correct. In contrast, the principal quantum number defines the size and energy of the orbit, while the magnetic quantum number specifies the orientation of the electron cloud within a sub-shell.
Hence, the answer is the option (1).
Practice more questions from the link given below
Frequently Asked Questions (FAQs)
Thomson's model (1904) proposed that an atom is a uniform sphere of positive charge, with negatively charged electrons embedded in it, much like plums in a pudding. The positive and negative charges were equal in magnitude, making the atom electrically neutral. It was discarded because it couldn't explain the results of Rutherford's alpha-particle scattering experiment, which showed that the positive charge and most of the mass of an atom were concentrated in a tiny central nucleus.
The three fundamental subatomic particles are:
- Electrons (e⁻): Negatively charged (-1 unit), negligible mass (9.109 × 10⁻³¹ kg). Found in orbitals outside the nucleus.
- Protons (p⁺): Positively charged (+1 unit), mass approximately 1 atomic mass unit (amu) (1.672 × 10⁻²⁷ kg). Found in the nucleus.
- Neutrons (n⁰): No charge (neutral), mass approximately 1 amu (1.674 × 10⁻²⁷ kg), slightly heavier than a proton. Found in the nucleus.
An atom is the fundamental building block of all matter. It is the smallest unit of an element that retains the chemical identity of that element. Atoms are incredibly tiny and are made up of even smaller particles.
The atomic number (Z) is the number of protons in an atom's nucleus. It is the most fundamental property of an atom because it uniquely identifies an element. All atoms of a given element have the same atomic number (e.g., every carbon atom has 6 protons, so its atomic number is 6).
Atoms are composed of three primary subatomic particles:
- Protons: Positively charged particles.
- Neutrons: Neutrally charged particles (no charge).
- Electrons: Negatively charged particles.
Bohr’s main postulates:
- Electrons move in quantized orbits with fixed angular momentum: L=nh2π (where n = orbit number).
Energy is emitted/absorbed when electrons jump between orbits ( ΔE=hν )
Orbits are stable ("stationary states")—no energy loss while in an orbit.
Bohr proposed (1913) that electrons orbit the nucleus in fixed, circular paths called stationary orbits (or shells) without radiating energy. Each orbit corresponds to a specific energy level.
| Particle | Charge (Coulomb) | Mass (kg) |
|---|---|---|
| Electron | -1.6 × 10⁻¹⁹ | 9.1 × 10⁻³¹ |
| Proton | +1.6 × 10⁻¹⁹ | 1.67 × 10⁻²⁷ |
| Neutron | 0 | 1.67 × 10⁻²⁷ |
Limitations of Rutherford’s model:
It couldn’t explain the stability of atoms (since accelerating electrons should emit energy and collapse into the nucleus).
It didn’t explain the line spectra of elements.