Cloning Vector: Definition, Types, Examples, Diagram, Technique
Cloning vectors are small, self-replicating DNA molecules that play a crucial role in genetic engineering. They carry an independent origin of replication and are used to insert foreign DNA fragments into host cells for cloning. These vectors are essential tools in biotechnology and its application, especially for gene manipulation and amplification in various organisms.
This Story also Contains
- What is a Cloning Vector?
- Types of Cloning Vectors
- Key Features of Cloning Vectors
- Mechanism of Cloning Using Vectors
- Applications of Cloning Vectors
- Advances in Cloning Vector Technology
- MCQs on Cloning Vectors
- Frequently Asked Questions (FAQs)
- Recommended Video on Cloning Vectors and Their Types

In recombinant DNA technology, cloning vectors enable the creation of genetically modified organisms by allowing the transfer and expression of desired genes. This has led to significant advancements in fields such as medicine, agriculture, and industry. From producing insulin to developing pest-resistant crops, cloning vectors continue to drive progress in biotechnology through innovative applications.
What is a Cloning Vector?
Cloning vectors are basic tools in genetic engineering and biotechnology. They are vehicles that introduce foreign genetic material into host cells. In simple words, cloning vectors help transfer the foreign material into the host cell. These tools help scientists manipulate the genes and thereafter enable recombinant DNA, leading to further research work in many fields, including medicine and agriculture.
Cloning vectors were discovered back in the 1970s when recombinant DNA technology appeared. The first vectors, for example, plasmid pBR322, were designed for replication inside bacterial cells. It thus signifies the beginning of modern genetic engineering.
Essential Features of Cloning Vectors
Origin of Replication (Ori): Using this sequence, the vector can replicate independently in the host cell.
Selectable Markers: This part contains a gene for antibiotic resistance or other markers (for example, ampR for resistance to ampicillin or lacZ for blue-white screening) to identify cells harbouring the vector.
Multiple Cloning Sites (MCS): Small DNA sequences comprising various restriction sites for inserting foreign DNA fragments.
Commonly Asked Questions
A cloning vector is a small DNA molecule, typically a plasmid or virus, used to carry foreign DNA into a host cell for replication and expression. It serves as a vehicle to introduce and propagate genetic material in a target organism.
Types of Cloning Vectors
Cloning vectors are classified based on their structure and the type of host cells they are used in. Different vectors are chosen depending on the size of the DNA fragment to be inserted and the purpose of the cloning experiment. Types of cloning vectors are:
Plasmid Vectors
Plasmid vectors are small circular DNA molecules, which replicate extensively in cloning and gene expression in bacteria. Examples include pBR322 and pUC19.
Bacteriophage Vectors
They are vectors derived from bacteriophages and are used to clone larger DNA fragments. An example is Lambda phage.
Cosmid Vectors
They are cosmid vectors, that is, the vectors that combine plasmids and bacteriophages, allowing them to carry DNA pieces up to 45 kilobases (kb) in size. An example is pWE15.
BAC (Bacterial Artificial Chromosome) Vectors
They are large plasmids that can clone very large DNA fragments between 100 and 300 kilobases. An example is pBAC108L.
YAC (Yeast Artificial Chromosome) Vectors
They are linear vectors carrying very large DNA fragments up to 1 megabase in yeast cells. An example is pYAC4.
Phagemid Vectors
They are plasmid-phage hybrid vectors and are utilised for the production of single-stranded DNA. Examples include pBluescript and pGEM.
Comparison of Different Cloning Vectors
Type of Cloning Vector | Description | Examples |
Plasmid Vectors | Small, circular DNA molecules are used for cloning and gene regulation and expression in bacteria. | pBR322, pUC19 |
Bacteriophage Vectors | Derived from bacteriophages, used for cloning larger DNA fragments. | Lambda phage |
Cosmid Vectors | A hybrid of plasmid and bacteriophage is used for cloning larger fragments (up to 45 kb). | pWE15 |
BAC Vectors | Large plasmids are used for cloning very large DNA fragments (100-300 kb). | pBAC108L |
YAC Vectors | Linear vectors that can clone very large DNA fragments (up to 1 Mb) in yeast cells. | pYAC4 |
Phagemid Vectors | A hybrid of plasmid and phage, useful for single-stranded DNA production. | pBluescript, pGEM |
Commonly Asked Questions
Plasmid vectors are circular DNA molecules that replicate independently in bacterial cells, while viral vectors are derived from viruses and can infect a wider range of host cells, including eukaryotes. Plasmids are easier to manipulate but have size limitations, whereas viral vectors can carry larger DNA inserts but may trigger immune responses.
Non-viral vectors, such as liposomes or nanoparticles, offer improved safety profiles and can carry larger DNA payloads compared to viral vectors. However, they generally have lower transfection efficiency and shorter duration of gene expression. Benefits include reduced immunogenicity and easier large-scale production.
Minicircle vectors are small, circular DNA molecules devoid of bacterial sequences. They offer improved safety and higher transgene expression compared to traditional plasmids, as they lack antibiotic resistance genes and other bacterial elements that can trigger immune responses or silencing in mammalian cells.
CRISPR-Cas9 compatible vectors are designed to express guide RNAs and Cas9 endonuclease, facilitating targeted genome editing. They often include features for easy guide RNA cloning and may incorporate inducible or tissue-specific promoters, enhancing the precision and control of gene editing experiments.
Vectors for CRISPRa or CRISPRi need to express a catalytically inactive Cas9 (dCas9) fused to transcriptional activators or repressors, along with guide RNAs. Key considerations include the choice of activation/repression domains, promoter strength, and delivery method to ensure efficient modulation of target gene expression without off-target effects.
Key Features of Cloning Vectors
Cloning vectors must have certain essential features like an origin of replication, selectable markers, and restriction sites for insertion of foreign DNA. These features help ensure that the DNA is successfully inserted and replicated inside the host organism. A number of the key features of cloning vectors that make them suited to genetic engineering include:
Origin of Replication (Ori)
Ori is the origin of replication, which is critical for the replication of vectors within a host cell. The replication mechanism and the plasmid copy number are determined by different Ori sequences, such as ColE1 and pMB1.
Selectable Markers
Selectable markers are the genes carried on vectors that help identify cells which have taken up the vector. The common ones include antibiotic resistance genes, such as ampR (ampicillin resistance) and kanR (kanamycin resistance), among others, and LacZ to assist in the blue-white screening.
Multiple Cloning Sites (MCS)
The MCS contains many restriction sites within the plasmid, enabling the insertion of foreign DNA fragments. With this, it is possible not only to make multiple clones but also to clone specific genes into the vector.
Promoter Regions
These are sequences that initiate the process of transcription of the inserted gene and are useful in gene expression studies.
Reporter Genes
For example, GFP is used as a reporter gene to make the identification of successful transformations easier through visual means.
High Copy Number
Vectors with high copy numbers yield a lot of DNA. This is especially useful when a large DNA yield is needed, for example, in large-scale experiments.
Commonly Asked Questions
An ideal cloning vector should have: 1) Origin of replication for independent DNA synthesis, 2) Multiple cloning sites for easy insertion of foreign DNA, 3) Selectable markers for identifying transformed cells, 4) Small size for efficient transformation, and 5) High copy number for increased yield of cloned DNA.
A multiple cloning site (MCS) is a short segment of DNA containing recognition sequences for multiple restriction enzymes. It's important because it provides flexibility in inserting foreign DNA, allowing researchers to choose from various restriction sites for efficient and precise cloning.
Cosmids are hybrid vectors that contain plasmid origins of replication and antibiotic resistance genes, along with phage packaging signals. This allows them to be packaged into phage particles for efficient infection of host cells, while also replicating as plasmids once inside the cell, combining advantages of both vector types.
A nuclear localization signal (NLS) is a short amino acid sequence that directs proteins to the cell nucleus. In eukaryotic expression vectors, including an NLS can ensure that the expressed protein is transported to the nucleus if required for its function, enhancing the effectiveness of the cloned gene.
Affinity tags are short peptide sequences added to the expressed protein to facilitate purification. Common tags like His-tag or FLAG-tag allow for easy one-step purification using affinity chromatography, simplifying the protein isolation process.
Mechanism of Cloning Using Vectors
The cloning process involves cutting both the vector and the foreign DNA with the same restriction enzyme, followed by ligation. The recombinant DNA is then introduced into a host cell where it multiplies and expresses the desired gene. Some of the major steps are discussed below:
Insertion of Foreign DNA
The process of ligation of the vector incorporates foreign DNA into the vector. Cut sites by the restriction enzymes allow for both the vector and the foreign DNA to be cut and joined together.
Ligation of Foreign DNA into a Vector
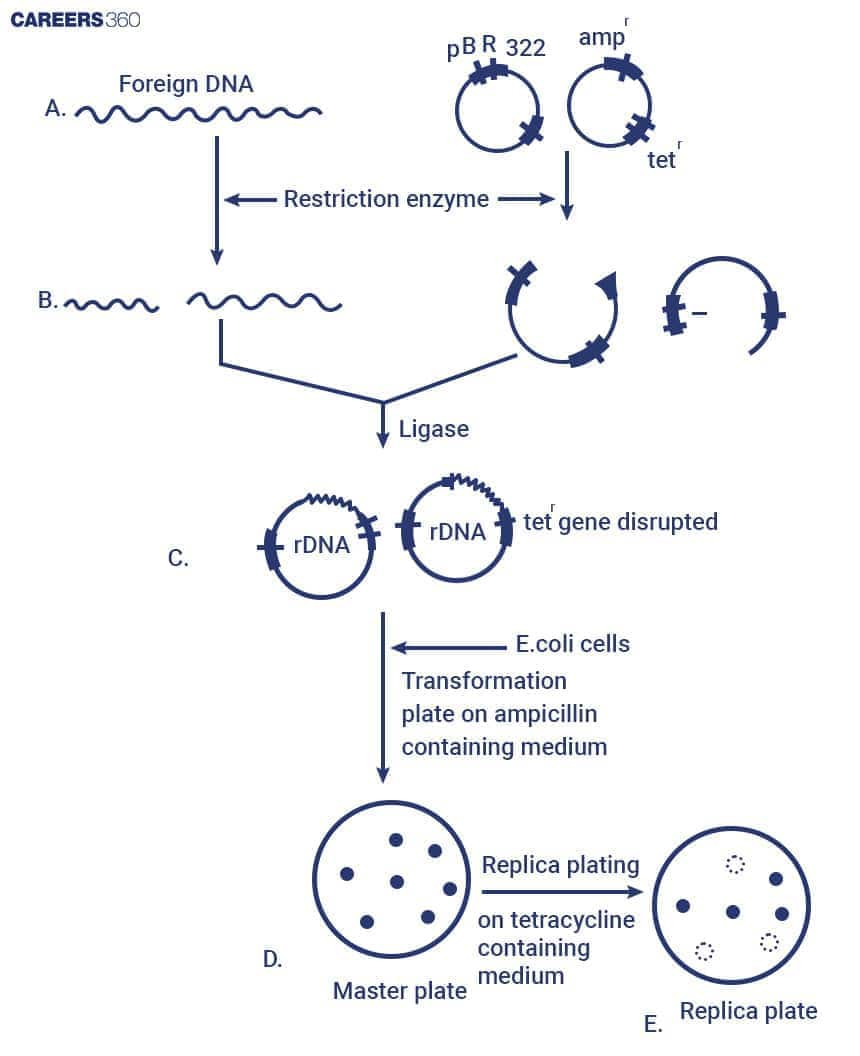
Transformation and Selection
Transformation refers to the process of introducing the recombinant vector into host cells. The methods used are heat shock, electroporation, and chemical transformation.
Commonly Asked Questions
The origin of replication (ori) is a specific DNA sequence that allows the vector to replicate independently of the host cell's chromosome. It ensures that the vector and its inserted DNA are copied and maintained in the host cell population, enabling the propagation of the cloned genes.
Selectable markers, typically antibiotic resistance genes, allow researchers to identify and select cells that have successfully taken up the vector. When grown in media containing the specific antibiotic, only transformed cells carrying the vector (and thus the resistance gene) will survive, enabling easy isolation of successfully cloned cells.
A promoter in an expression vector is a DNA sequence that initiates and regulates transcription of the cloned gene. It determines when and how much the gene is expressed, allowing researchers to control protein production in the host organism.
Episomal vectors replicate independently of the host chromosome and do not integrate into the genome. This reduces the risk of insertional mutagenesis but may result in less stable gene expression. Integrating vectors, on the other hand, insert their DNA into the host genome, potentially offering more stable, long-term expression.
Self-replicating RNA vectors, derived from alphaviruses, can replicate their RNA genome in the cytoplasm of host cells. They offer rapid and high-level transient gene expression without the need for nuclear entry or genome integration, making them useful for vaccine development and gene therapy applications.
Applications of Cloning Vectors
Cloning vectors are widely used in genetic engineering to produce proteins, study gene functions, and develop vaccines. They also play a crucial role in various research and industrial applications related to biotechnology. Some of the basic uses of cloning vectors is discussed below:
Gene Cloning
Selection refers to identifying cells that have successfully taken up the vector. This is usually done using methods such as antibiotic resistance or reporter genes.
Protein Expression
Cloning vectors make it possible to multiply specific genes for detailed studies on function and structure.
Gene Therapy
Vectors enable the production of so-called recombinant proteins, which are indispensable in research, medicine, and industry.
Genomic Library Construction
Vectors deliver therapeutic genes to target cells in gene therapy and are one of the only hopes for treating several human genetic disorders. Genomic libraries are collections of DNA fragments that are used in constructing cloning vectors representing the entire genome content of an organism. These libraries are one of the most essential parts of sequencing projects on the genome.
Commonly Asked Questions
The lac operon in E. coli vectors provides an inducible system for gene expression control. It allows researchers to regulate the expression of cloned genes by adding IPTG (a lactose analog), enabling fine-tuned control over protein production.
Suicide vectors are designed to replicate in one bacterial species but not in the target species. They're used to introduce genes into bacterial chromosomes through homologous recombination, allowing for gene knockout studies or stable gene integration without maintaining the vector.
Binary vectors consist of two plasmids: one containing the gene of interest and another with virulence genes. They work with Agrobacterium tumefaciens to transfer DNA to plant cells. The separation of these components enhances safety and efficiency in plant genetic engineering.
Temperature-sensitive replicons allow vector replication only at specific temperatures. This feature can be used to control plasmid copy number, study essential genes by conditional replication, or facilitate the curing of plasmids from bacterial populations by changing growth temperature.
Introns can enhance gene expression in eukaryotic cells through a process called intron-mediated enhancement. They can increase mRNA stability, improve nuclear export of mRNA, and sometimes contain regulatory elements that boost transcription, leading to higher protein yields.
Advances in Cloning Vector Technology
Modern cloning vectors have been improved for better efficiency, such as higher insertion capacity, and multiple cloning sites. These advancements help in faster and more accurate genetic manipulation.
Synthetic Vectors: The recent advancement in this area has been the development of synthetic vectors specially designed for particular applications, thus being more efficient and versatile.
CRISPR/Cas9 Vectors: These vectors are of prime importance in genome editing since they allow for precision in the modifications of DNA sequences.
Latest Innovations: On the other hand, continuous research is rendering newer and more efficiency-specific, user-friendly vectors, thus allowing more possibilities in genetic engineering.
Commonly Asked Questions
A shuttle vector is designed to replicate in two different host species, typically a prokaryote (like E. coli) and a eukaryote. It's used when researchers need to manipulate DNA in a bacterial system for ease of cloning, then transfer it to a eukaryotic host for expression or further study.
BAC (Bacterial Artificial Chromosome) and YAC (Yeast Artificial Chromosome) vectors can carry much larger DNA inserts (up to 300 kb for BACs and 1 Mb for YACs) compared to plasmid vectors (typically up to 10 kb). They are used for cloning large genomic fragments or entire genes with their regulatory elements.
Inducible promoters allow researchers to control when gene expression occurs by adding a specific inducer molecule. This is useful for expressing proteins that might be toxic to the host cell, as expression can be delayed until the desired time, or for studying gene function by turning expression on or off.
Expression vectors are designed specifically for protein production, containing regulatory elements like strong promoters and ribosome binding sites to drive high-level expression of the cloned gene. Standard cloning vectors focus on DNA replication and may lack these expression-enhancing features.
A poly-A tail is a sequence of adenine nucleotides added to the 3' end of mRNA in eukaryotes. In expression vectors, it enhances mRNA stability and translation efficiency, leading to improved protein production in eukaryotic host cells.
MCQs on Cloning Vectors
Q1. Which of the following is not a feature of the plasmids?
Independent replication
Circular structure
Transferable
Single-stranded
Correct answer: 4) Single-stranded
Explanation:
Plasmids - A plasmid is a small, circular standard DNA molecule; that has the power of self-replication. They usually exist in bacteria and some lower eukaryotes. They have antibiotic-resistance genes. Plasmids provide genetic advantages like antibiotic resistance. Plasmids are double-stranded circular structures.
Hence, the correct option is 4) Single-stranded.
Q2. Assertion (A): Integration into the host cell's chromosome is a common outcome for plasmids used as cloning vectors following transformation.
Reason (R): Plasmids undergo fragmentation or disintegration upon transformation.
Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A).
Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of Assertion (A).
Assertion (A) is true, but Reason (R) is false.
Both Assertion (A) and Reason (R) are false.
Correct answer: 4) Both Assertion (A) and Reason (R) are false.
Explanation:
Assertion (A) claims that plasmids as cloning vectors usually integrate into the host cell's chromosome after transformation. However, this statement is not true. Plasmids used as cloning vectors typically remain as separate, extrachromosomal entities within the host cell and do not integrate into the chromosome unless specific mechanisms or techniques are employed to facilitate integration.
However, Reason (R) is false in suggesting that plasmids get disintegrated after transformation. Plasmids typically do not disintegrate or fragment upon transformation. Instead, they persist as separate, extrachromosomal elements within the host cell, unless they undergo integration into the chromosome.
Hence, the correct answer is option 4) Both Assertion (A) and Reason (R) are false.
Q3. Commonly used vectors for human genome sequencing are
T - DNA
BAC and YAC
Expression Vectors
T/A Cloning Vectors
Correct answer: 2) BAC and YAC
Explanation:
Separated fragments are coupled to vectors like BAC (bacterial artificial chromosome) and YAC (yeast artificial chromosome) and cloned inside the host bacterium or yeast to be amplified during the procedure. A designed DNA molecule called a bacterial artificial chromosome (BAC) is used to replicate DNA sequences in bacterial cells, such as E. coli. When it comes to DNA sequencing, BACs are frequently utilized. Therefore, BAC and YAC are the proper answers.
Hence, the correct answer is Option 2) BAC and YAC.
Also Read:
Frequently Asked Questions (FAQs)
Q1. What is a cloning vector?
A cloning vector is a small piece of DNA used to transfer foreign genetic material into a host cell for cloning.
Q2. What are the four types of cloning vectors?
The four main types of cloning vectors are plasmids, bacteriophages, cosmids, and artificial chromosomes.
Q3. What are the two main types of cloning?
The two main types are reproductive cloning and therapeutic cloning.
Q4. What are the two features of a cloning vector?
A cloning vector must have an origin of replication (Ori) and a selectable marker like an antibiotic resistance gene.
Q5. What is the difference between a YAC and a BAC?
YAC (Yeast Artificial Chromosome) is used in yeast cells, while BAC (Bacterial Artificial Chromosome) is used in bacteria and is more stable.
Recommended Video on Cloning Vectors and Their Types
Frequently Asked Questions (FAQs)
Large vectors can be difficult to manipulate due to their size, have lower transformation efficiency, and may be less stable in host cells. They can also be challenging to isolate and purify in high yields, and may undergo rearrangements or deletions during propagation.
The choice depends on the protein's complexity, required post-translational modifications, expression level needed, and intended use. Prokaryotic systems (like E. coli) are simpler and faster but may not properly fold complex eukaryotic proteins. Eukaryotic systems (like yeast or mammalian cells) can perform more complex modifications but are slower and more expensive.
Site-specific recombination systems allow for rapid and efficient transfer of DNA sequences between vectors without traditional restriction enzyme cloning. This speeds up the cloning process, enables high-throughput applications, and facilitates the use of multiple vector types for different experimental purposes.
Inducible degradation systems, like the auxin-inducible degron (AID) system, allow researchers to rapidly and reversibly deplete a protein of interest. This enables the study of protein function by observing the effects of its sudden removal, providing temporal control over protein levels in the cell.
Codon optimization involves adjusting the DNA sequence to use codons preferred by the host organism without changing the amino acid sequence. This can significantly enhance protein expression levels, especially when expressing genes from organisms with different codon usage biases.
Chromatin insulators are DNA sequences that can shield genes from the effects of nearby chromatin structure or regulatory elements. In mammalian expression vectors, they can help maintain consistent transgene expression by preventing silencing effects from surrounding chromatin, enhancing long-term stability of gene expression.
Cell-penetrating peptides are short amino acid sequences that can traverse cell membranes. When incorporated into non-viral vectors, they enhance cellular uptake of DNA or RNA, improving transfection efficiency and potentially allowing for targeted delivery to specific cell types or subcellular locations.
Matrix attachment regions are DNA sequences that bind to the nuclear matrix. When included in mammalian expression vectors, MARs can enhance and stabilize transgene expression by creating independent chromatin domains, reducing position effects, and potentially increasing the probability of integration into active chromatin regions.
Split-intein vectors express two parts of a protein separately, each fused to complementary intein fragments. Upon expression, the intein fragments associate and splice the two protein parts together. This system is useful for expressing toxic proteins, studying protein-protein interactions, or creating circular proteins.
Key challenges include ensuring efficient processing of short hairpin RNAs (shRNAs) to functional siRNAs, minimizing off-target effects, and maintaining stable expression over time. Vector design must consider promoter choice, shRNA sequence optimization, and delivery method to achieve effective and specific gene silencing.